Top Links
Journal of Surgery and Operative Care
ISSN: 2455-7617
Inguinal Hernia. A Review
Copyright: © 2016 Onuigbo WIB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Background: Inguinal hernia is a common surgical problem, but it can present a surgical dilemma for the skilled surgeon when it exhibits some unusual contents. While watchful waiting is an acceptable strategy for minimally symptomatic hernias, the definitive treatment of inguinal hernias, regardless of their origin or type, is surgical repair. Surgical repair is the only chance a surgeon has to deal with any abnormal content that may arise. This paper is intended to make readers, especially young surgeons, surgical residents and general practitioners aware of current thinking in the management of inguinal hernias.
Methods: Publications in English language from 1959 up to 2015 were obtained from both reprint requests and by searching Pubmed database. Data extracted included authors, country, year of publication, age and sex of patients, epidemiology, geographical distribution, pathophysiology, risk factors, clinical features, investigations, types of surgical treatment, and unusual findings in inguinal hernias.
Results: The pathophysiology explains the higher incidence of inguinal hernias in males than females, while the aetiology explains the physiological background for the different modern methods for repair of inguinal hernias and the reason for recurrence of hernias using the older sutured repairs. In addition, some other unexpected benign and malignant lesions were found to also present in inguinal hernias.
Recommendations: Up to date knowledge of these findings is important for proper repair of inguinal hernias to reduce recurrence rates, and careful handling of these unusual contents of inguinal hernias.
Keywords: Inguinal hernia; Epidemiology; Risk factors; Surgical operations; Unusual findings
Hernias are among the oldest recorded afflictions of mankind and they are most commonly seen in the groin [1,2]. A hernia is defined as a protrusion, bulge or projection of an organ or a part of an organ through the body wall that normally contains it. In an inguinal hernia the protrusion occurs through the inguinal canal. Groin hernias are the most common conditions for which primary care physicians refer patients for surgical management [3]. Approximately 96% of groin hernias are inguinal and 4% are femoral [3]. Inguinal hernia repair is therefore one of the most common operations in surgical practice [2]. They constitute an important public health problem [4]. In spite of its great incidence, hernias can pose a surgical dilemma, even for the skilled surgeon, because many pathologic entities can masquerade as inguinal hernia. The unexpected hernial content and pseudohernias constitute some of these cases [5]. The incidence of inguinal hernia is unknown, but about 500,000 cases come to medical attention each year [6]. This paper is intended to make readers, especially young surgeons surgical residents and general practitioners, aware of current thinking in the management of inguinal hernias.
Publications in English language on inguinal hernias from 1959 to 2015 were obtained from both reprint requests and by searching PubMed database. Data extracted from these papers included authors, country, year of publication, age and sex of patients, epidemiology, pathogenesis, risk factors for development of inguinal hernias, racial distribution, presenting symptoms, surgical treatment and unusual findings in inguinal hernia surgery.
Although inguinal hernias occur in both sexes, they are more common in men compared with women; and more in whites compared with non-whites among United States adults [7]. In general, inguinal hernia affects all ages, but the incidence increases with age [8]. With respect to gender, women manifest inguinal hernia at a later age, their peak age range at presentation being 40 to 60 years of age, with median age of 60 to 79 years, unlike that of men, which is 10 years earlier [9]. Inguinal hernia is bilateral in up to 20% of affected adults [10]. It is more common on the right than on the left, the ratio being 2:1, possibly because of the later descent of the right testicle and the associated patent processus vaginalis. Appendicectomy scar has also been suggested as another reason for more common occurrence on the right side [11,12]. The prevalence of hernia is significantly higher in the presence of varicose veins, in men who reported symptoms of prostatic hypertrophy, in the presence of haemorrhoids and, among lean men [13]. These associations may reflect the role of increased abdominal pressure. The prevalence of hernia is low in the presence of overweight or adiposity, suggesting that obesity is a protective factor [13, ].
Although the incidence and prevalence of inguinal hernias worldwide is unknown, it is estimated, that in the United States, approximately 4.5 million people have inguinal hernia [15] while in Jerusalem, it was documented as 18 per 100 men aged 25 and over [13].
Little is known about the epidemiology of inguinal hernia in resource poor settings, however the prevalence of inguinal hernia in Tanzanian adults is 5.36%, and an estimated 12.09% of men had hernias [16]. It has been argued that, since surgery is the main elective treatment for repairing inguinal hernia, surgical audit data can be considered a reasonable indicator of incidence/prevalence rates. In England for instance, about 70,000 inguinal hernia repairs are performed each year, and this constitutes approximately 0.14% of the population each year [17]. These statistics give an insight into the burden of this disease.
Traditionally inguinal hernias, are classified as (a) direct (b) indirect, and (c) combined hernias, otherwise known as pantaloon or Romberg or saddle bag hernias, depending on their relationship to the inferior epigastric vessels. Direct inguinal hernias occur medial to the inferior epigastric vessels when abdominal contents herniate through a weak spot in the fascia of the posterior wall of the inguinal canal, which is formed by the transversalis fascia. Indirect inguinal hernias occur when abdominal contents protrude through the deep inguinal ring, lateral to the inferior epigastric vessels. This may be caused by failure of embryonic closure of the processus vaginalis. In the combined variety, hernia sacs are on both sides of the inferior epigastric vessels. A direct inguinal hernia is less common (~25–30% of inguinal hernias) and usually occurs in men over 40 years of age [18]. The European hernia society [EHS] has an official classification for groin hernias which is good, simple and easy to remember [19]. This classification mentions both anatomical location and size of the hernia orifice as seen intra-operatively. It localizes the hernia anatomically as L = lateral, M = medial, F = femoral and measures the size of the hernia orifice using the tip of the index finger which is about 1.5-2cm. This is registered on the table as 1 (≤1 finger), 2 (1-2 fingers), and 3 (≥3 fingers). Thus a hernia orifice of 2.5 cm is depicted as a size 2 hernia. This dimension is reported to be identical to the length of branches of a pair of most laparoscopic graspers, dissectors, or scissors enabling the surgeon to use the same classification during laparoscopic surgery [19]. In this classification, combined hernias are ticked in the appropriate boxes (Table 1).
A hernia can be classified as reducible or irreducible. A reducible hernia is one in which the contents can be pushed back into the abdomen by putting manual pressure on it. An irreducible/incarcerated hernia is one in which the contents cannot be pushed back into the abdomen by applying manual pressure. Irreducible hernias are further classified into obstructed and strangulated hernias. An obstructed hernia is one in which the lumen of the herniated part of intestine is obstructed whereas a strangulated herniais one in which the blood supply of the hernia contents is compromised, thus, leading to ischemia. The lumen of the intestine may or may not be patent. No matter which classification system is used the type of hernia should be recorded according to intraoperative findings.
In men, indirect hernias follow the same route as the descending testes, which migrate from the abdomen into the scrotum during the development of the urinary and reproductive organs. The larger size of their inguinal canal, and deep ring, which transmitted the testicle and accommodates the structures of the spermatic cord, might be one reason why men are many times more likely to have an inguinal hernia than women [20]. An indirect hernia is generally believed to have a congenital component which requires a potential hernia sac, i.e. the processus vaginalis. After the descent of the foetal testis into the scrotum from the retroperitoneum, the processus vaginalis should obliterate [3]. If the processus vaginalis is not obliterated, fat or bowel may get into it.
The pinchcock action of the internal ring musculature during abdominal muscular straining prohibits protrusion of the intestine into a patent processus. Muscle paralysis or injury can disable the shutter effect. In addition, the transversus abdominis aponeurosis flattens during tensing, thus reinforcing the inguinal floor. A congenitally high position of the aponeurotic arch may preclude the buttressing effect. Neurapraxic or neurolytic sequelae of appendectomy or femoral vascular procedures may increase the incidence of hernia in these patients [11].
Inguinal hernia is a known complication after radical retropubic prostatectomy, whether it is open or laparoscopic, and has been reported in up to 15-21% of patients. It has also been suggested that all lower mid line incisions have the same increased risk, as they disrupt the shutter mechanism and thus increase the risk of an inguinal herniation. [21,22].
The aetiology of indirect hernias is largely explainable in terms of the embryology of the groin and of testicular descent. An indirect inguinal hernia is therefore a congenital hernia, regardless of the patient’s age. It occurs because of protrusion of an abdominal viscus into an open processus vaginalis. More generally, any condition that increases the pressure in the intra-abdominal cavity may contribute to the formation of a hernia. Direct inguinal hernia is caused by weakness in the transversalis fascia area of the Hesselbach’s triangle. This is a triangle which is bounded laterally by the inferior epigastric vessels, medially by the lateral border of the rectus abdominis muscle and inferiorly by the inguinal ligament.
Abnormal collagen metabolism is thought to play an important role in the development of primary inguinal hernia. An increase of type III collagen, (the thin isolated fibres) leads to a decreased ratio of type I, (the thick fibre bundles) to type III collagen. This alters the physical properties and the strength of the collagen matrix of the abdominal wall, and may predispose individuals to development of inguinal hernias [23].
In addition to male sex and increased age, a major risk factor for a groin hernia is a family history of groin hernias [24]. Other conditions reported to be associated with increased risk for both sexes include smoking, which causes a defective connective tissue metabolism, and chronic obstructive pulmonary disease [25]. Among women, rural residence, greater height, and umbilical hernia were in additional risk factors for inguinal herniation [26]. Lower body-mass index, high intra-abdominal pressure, collagen vascular disease, thoracic or abdominal aortic aneurysm, patent processus vaginalis, history of open appendectomy, and peritoneal dialysis are also risk factors [25].
Patients with matrix metalloproteinase (MMP) abnormalities, (increased MMP-2 expression and MMP tissue inhibitor 2 activity) such as Ehlers–Danlos, Marfan’s, Hurler’s, and Hunter’s syndromes, also have increased risks of having direct hernias. Matrix metalloproteinase is a family of proteolytic enzymes that degrade protein components of the extra-cellular matrix. Such increased proteolytic activity may cause weakness in structural tissue and abnormal connective-tissue homeostasis [27].
Whether heavy lifting is also a risk factor remains controversial. A recent systematic review showed data concerning the relationship between occasional heavy lifting, repeated heavy lifting, or a single strenuous lifting episode and the development of a groin hernia to be inconclusive [25]. Of note, is that weight lifters do not have an increased incidence of inguinal hernias [14].
A bulge in the area on either side of the pubic bone indicates an inguinal hernia. There may be a burning, gurgling or aching sensation at the bulge, or pain or discomfort in the groin, especially when bending over, coughing or lifting. As the hernia progresses, contents of the abdominal cavity, such as the stomach, small bowel, colon, and liver, can descend into the hernia [28]. Occasionally, pain and swelling around the testicles occurs, when the protruding intestine descends into the scrotum.
An incarcerated hernia may be associated with inability to manipulate the hernia through the fascial defect. Pain, nausea, and vomiting, indicate bowel obstruction while persistence of pain and tenderness of an incarcerated hernia indicate strangulation. In addition, there may be systemic toxicity secondary to ischemic bowel.
Most early inguinal hernias can be diagnosed by careful physical examination. The physical examination should begin by carefully inspecting the inguinal areas for bulges while the patient is standing. Then, the patient should be asked to cough or strain down (i.e., Valsalva manoeuvre) while the physician observes for bulges. It is more challenging to diagnose a hernia in female patients. Diagnosis is based not only on inspection but also by palpation with an open hand over the groin area which might feel a bulge or detect the impulse of a hernia during a Valsalva maneuver [26] or with a finger introduced into the scrotum.
Although imaging is rarely needed to diagnose a hernia, it may be useful in certain clinical situations (e.g., suspected sports hernia; recurrent hernia; uncertain diagnosis; surgical complications, especially chronic pain) [26]. Young surgeons should however note that its use is limited to a small number of patients, because in the vast majority of patients, clinical examination is sufficient. Ultrasound may be useful in diagnosing inguinal hernias in patients who report symptoms but do not have a palpable defect. It may also be helpful in differentiating an incarcerated hernia from a pathologic lymph node or other cause of a firm, palpable mass [3]. Magnetic resonance imaging (MRI) provides the most sensitive detection of a hidden hernia in a patient with clinical suspicion for hernia [29].
There is currently no medical recommendation about how to manage an inguinal hernia condition, due to the fact that until recently, elective surgery used to be recommended for all inguinal hernias. The reason for this recommendation is the feared risk of complications such as incarceration or strangulation [25]. However in most cases, surgical repairs are not carried out to prevent strangulation, but because of patients’ request, to relieve discomfort [30]. Watchful waiting therefore is a recommended reasonable option, especially for minimally symptomatic hernias, (Figure 1) due to the significant risk of chronic post herniorraphy pain (>10%), and the low risk of incarceration (<0.2% per year) [25].
Most inguinal hernia repairs can be performed safely, accurately and cost-effectively using local anesthesia, through an open anterior approach. Hernia recurrence rates of less than 4% have been reported for herniorrhaphies performed without prosthetic mesh by skilled surgeons [31]. Hernia repair using prosthetic mesh would be a good choice in the patient with a direct hernia or in the older patient with a longstanding hernia and attenuated fascia. Recurrent hernias repaired with classical herniorrhaphy not utilizing mesh have a reported recurrence rate of approximately 23% at three years [32]. For this reason, recurrent hernias are best managed with open anterior or posterior mesh repair and laparoscopic repair.
Open techniques for inguinal hernia repair include (a) tension-free mesh repairs, [33] (b) tension-free suture repairs (Desarda) [34] and (c) the older method of tension suture repairs.
Meshes have reduced the rate of recurrence of hernias significantly, (Table 2) but some problems related to meshes have been reported [35]. A mesh has certain features like material, strength, elasticity, density, pore size. Standard polypropylene mesh is the most frequently used one. It is cheap, available, non-absorbable, and strong enough to avoid recurrence. Nevertheless, some actual problems with mesh use like foreign body sensation and chronic post-operative pain (Table 2 & 3) have created a conflict about standard polypropylene mesh [35]. Polyester mesh might be an alternative, but did not gain popularity as it can degrade with time, especially in infected areas [36].
Newer lighter meshes have been produced to overcome those problems but they are more expensive than the standard polypropylene mesh [37]. Pure polypropylene light mesh is the most economic option. There are also coated polypropylene meshes which attenuate the host response to the prosthesis, yet still provide the adequate strength for repair [38]. Fish oil, beta glucan and titanium have been used for coating [36]. When meshes are classified by density, a mesh with density >100gm/m2 is accepted as heavy, whereas a 35-50 gm/m2 density is classified as light weight, and light weight meshes are thought to improve patient comfort [36,39,40].
The Lichtenstein repair involves the placement of a flat mesh on top of the defect which strengthens the inguinal region. The basis for use of mesh is that the posterior wall of the inguinal canal is deficient and therefore, such weakened muscles or transversalis fascia should not be used for repair [33]. Permanent meshes are typically made of polypropylene or polyester. Complications of mesh use occasionally occur, and these include, chronic pain (varying from 10-50% depending on source), foreign-body sensation, stiffness, (Table 3) ischemic orchitis, testicular atrophy dysejaculation, anejaculation or painful ejaculation in around 12% of patients [41]. Ischemic orchitis, testicular atrophy are however not peculiar to mesh repairs. (Table 2) In the long term, polypropylene meshes face degradation, due to heat effects [42]. This increases the risk of stiffness and chronic pain, infection, adhesion formation, and erosion into intraperitoneal organs [42,43]. These occasional complications usually become apparent weeks to years after the initial repair, presenting as abscess [44], bowel obstruction [45,46] or in very few cases, intestinal perforation [47].
Traditional hernia surgery carries a high risk of chronic pain, (Table 2) and as many as 17% of patients can have significant pain for years. This high incidence is likely due to the location of the mesh used for this kind of surgery. It may also be related to nerve scarification, mesh contraction, chronic inflammation, or osteitis pubis. There are procedures that lower this chronic pain, e.g. the open preperitoneal repair, where the nerves responsible for the chronic pain are avoided, leading to a lower incidence of this problematic complication [48]. This procedure is done as a minimally invasive trans inguinal preperitoneal patch procedure (TIPP) or as trans rectus sheath extraperitoneal procedure (TREPP) under local anaesthesia [49,50]. This minimally invasive procedure allows extensive dissection in the preperitoneal space, including below Cooper’s ligament. It facilitates placement of the mesh without any need for suturing, allowing passive pressure of the peritoneal contents to keep the mesh in place [51]. Randomized clinical trials have shown significant differences in favour of TEP (total extraperitoneal) and TIPP compared with Lichteinstein with respect to controlling postoperative chronic pain and physical functioning [52,53].
Mosquito-net mesh: Meshes made of mosquito cloth, in coplymer of polyethylene and polypropylene have been used for low-income patients in rural India and Ghana [54,55]. Each piece costs $0.01 and the results are identical to those obtained using commercial meshes, in terms of infection and recurrence rate at 5 years [56].
Absorbable mesh: Biomeshes which are absorbable resulted from a search for a biomaterial that could address the problems associated with permanent synthetic mesh, including chronic inflammation and foreign body reaction, stiffness and fibrosis, and mesh infection. They are made from human or porcine dermis which have been processed to acellular, porous extracellular matrix scaffold of collagen and elastin. They are expensive, but can be used for repair in infected environment, like for an incarcerated hernia [57]. Moreover, they seem to improve comfort and presumably reduce the risk of inguinodynia [58]. Absorbable mesh will degrade and lose strength over time. It is not intended to provide long-term reinforcement to the repair site. As the material degrades, new tissue growth is intended to provide strength to the repair [59].
The Bassini technique is a “tension” repair, in which the edges of the defect are sewn back together, i.e. the conjoint tendon is approximated to the inguinal ligament and closed without any mesh. Interest in Bassini’s technique is now historical, but remains performed in some developing countries, if surgeons do not have knowledge of the tension-free repairs.
In McVay/Cooper’s ligament repair, the floor of the canal is reinforced by approximating the transversus abdominis aponeurosis and transversalis fascia to pectineal (Cooper’s) ligament medially from the pubic tubercle to the femoral vein. Lateral to this, the floor is restored by approximating the femoral sheath to the inguinal ligament. It is also used in femoral hernia repairs.
The Shouldice technique is a relatively difficult four layer reconstruction of fascia transversalis; however, it has relatively low reported recurrence rates in the hands of surgeons experienced with this method.
The Desarda technique is an emerging suture-based technique. This technique is tension-free, mesh-free, and pays attention to the surgical physiology of inguinal canal [34]. It uses the external oblique aponeurosis sutured to the inguinal ligament and internal oblique to reinforce the posterior wall of the inguinal canal [34]. It also gives similar results to Lichtenstein in terms of recurrence, with the significant benefit of not introducing permanent foreign-body material [60]. Other post-operative sequelae of the Desarda technique compared with the Lichtenstein are shown in Table 3. Like Desarda technique, the Guarnieri is another tension-free technique which pays attention to the surgical physiology of inguinal canal [61]. In this procedure where a mesh is not used, the inguinal canal is reinforced by overlapping the external oblique aponeurosis in a double breasted fashion.
This technique for repair of hernias has varying degrees of usage in different industrialized countries, and one of its disadvantages is that it needs surgeons highly experienced in laparoscopic hernia surgery [62]. Whereas its use is confirmed to be low in some industrialized countries like the United Kingdom and Japan, laparoscopic hernia repair has gained popularity in North America and some European countries, like Germany, accounting for 15-30% of hernia repairs in these countries. [35] Laparoscopic repairs are more expensive than open repairs. Whereas Hynes et al reported that laparoscopic repair costs an average of $638 more than open repair in North America [63], McCormack, et al. reported that in the UK, laparoscopic repair cost an extra 300-350 pounds per patient [17]. The two main laparoscopic inguinal hernia repairs are the totally extraperitoneal (TEP) and transabdominal preperitoneal patch (TAPP) repairs [64] and each is regarded as tension-free and requires the use of mesh.
When performed by a surgeon experienced in laparoscopic hernia repair, there are fewer complications than Lichtenstein, particularly less chronic pain and numbness and return to usual activities is faster [52,65,66]. In a randomized comparison of laparoscopic and Lichtenstein inguinal hernia repair, Eklund, et al. demonstrated that five years after surgery only a small proportion of patients still reported moderate to severe chronic pain. It was even less for those who had laparoscopic inguinal hernia repair [66].
With respect to recurrent hernia repairs, haematoma/seroma formation is less after laparoscopic hernia repair. However, operation time is longer and there appears to be a higher risk of complication rate, with respect to visceral and vascular injuries. Vascular injury is a relatively uncommon but nonetheless potentially disastrous adverse event. Lichtenstein repair has shorter operating time, and is less expensive [35]. Laparoscopic repair is thought to be a good option for bilateral hernia repair as both sides can be repaired via the same laparoscopic port sites [67].
It had been stated that unusual hernia contents or findings in the inguinal canal can pose a surgical dilemma [2]. A lipoma of the cord on round ligament is one such finding. It can cause groin pain in both sexes, but more so in females, probably due to the size of the internal ring in women. Such lipomas have occasionally been shown to grow very large, mimic irreducible hernia, and in males can grow into the scrotum to cause diagnostic confusion [2]. An inflamed swollen epiploic appendage of the sigmoid colon has been found in inguinal hernia sac. This epiploic appendagitis as it called, causes irritation symptoms and mass formation, without a cough impulse [2].
Herniation of the ovary is rare and occurs in <3% of hernias in women [68]. Entrapment of the adnexa in an indirect hernia is rare in adult women [2]. Most reported cases concern the paediatric population and 30% occurs in adolescents of reproductive age [2]. Complications include ovarian torsion, incarceration or salpingitis.
Although rare, a hernia sac may contain vermiform appendix; it is called an Amyand’s hernia and exceptionally, an acutely inflamed appendix which constitutes 0.07-0.13% of all cases of acute appendicitis [69]. Inflammation of the appendix is caused by pressure at the neck of the hernia. When the sac contains a Meckel’s diverticulum, it is called a Littre’s hernia, [2].
Benign tumors have been reported in the inguinal canal as lumps usually masquerading as inguinal hernias. Examples are Schwannoma [70], and benign mesothelial cyst; the latter could be confused with a malignant myxoma and may lead to the performance of radical cancer surgery [71].
Ectopic nephrogenic rests (ENR), which develop from a persisting nephrogenic blastema have been reported in the inguinal canal, but this is rare. Most of these were associated with patent processus vaginalis. Some undergo undergo neoplastic transformation into benign adenomas or Wilms tumor [72].
Ectopic adrenal tissue remnants are encountered incidentally in 1%-9.3% of children undergoing inguinal operations. These rests present as bright yellow nodules, and should be removed, as they may undergo marked hyperplasia or neoplastic changes [73].
Standard texts do not mention the occurrence of primary or metastatic tumors of the hernia sac, but they are seen in less than 0.4% of excised sacs, and most of them are metastatic [74]. These tumors have been classified as saccular or intrasaccular tumors, based on their relationship with the inguinal sac. Intra-abdominal malignancies like colonic cancers, presenting as inguinal hernias are known to occur. However, less than 1 out of 200 cases of these malignant colonic carcinomas is localized within an inguinal hernia, and have been known to cause colonic obstruction [74-76]. Liposarcoma makes up 7% of all paratesticular sarcomas, of which 12% occur in the inguinal canal [77].
Parts of the urinary tract can present as sliding hernias. Ureteral hernia occurs, though uncommonly in males without urinary symptoms [78]. Oruç, et al. indicated that there are 64 cases of inguinoscrotal herniation of the ureter in the English language literature [80]. The incidence of inguinal hernia containing urinary bladder is 0.36% [68]. It is important to be aware of these conditions to avoid damage these structures when managing this rare hernia [79].
Indirect inguinal hernia is a common finding in small infants. Early surgical intervention is usually recommended to avoid the development of obstruction. Infarcted testes have been found in hernia sac of such obstructed hernias [80]. Incomplete descent of the testis into the scrotum occurs in 2-8% of males at birth [81]. An atrophic, cryptorchid testis has been reported in the hernia sac of a 50 year old man [82]. One other uncommon content of inguinal hernia sac is an abscess. Collections of the infected ascites can remain in recesses of the peritoneal cavity after peritonitis. This may lead to inflammation and the formation of an abscess in the hernia sac [76].
Even though hernia is a common surgical problem, up to date knowledge of herniology is important for proper repair of inguinal hernias to reduce recurrence rate, and careful handling of these unusual contents of inguinal hernias, to avoid damage to some of these structures.
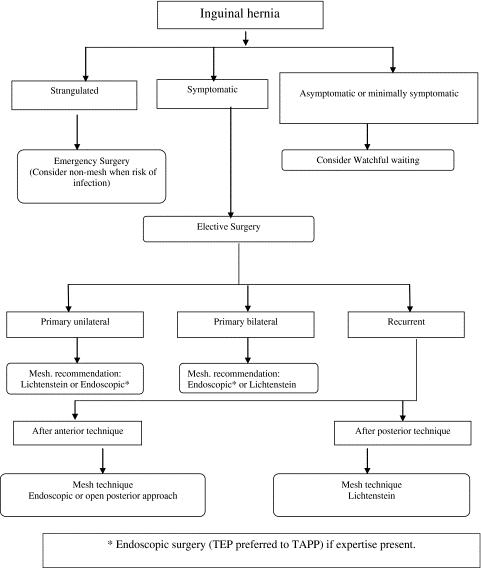 |
| Adapted from Simons M. P, et al. 2009. European Hernia Society guidelines on the treatment of inguinalhernia in adult patients Figure 1: Flow diagram for the treatment of inguinal hernia inmale adults |
EHS
Groin Hernia Classification |
Primary |
Recurrent |
|||
|---|---|---|---|---|---|
0 |
1 |
2 |
3 |
x |
|
L |
|||||
M |
|||||
F |
|||||
| Key: Primary = primary hernia, Recurrent = recurrent hernia, 0 = no hernia detectable, 1 = <1,5 cm (one finger), 2 = <3 cm (two fingers) Table 1: EHS Groin Hernia Classification |
|||||
Repair |
Lichtenstein |
PHS |
Darn |
Mesh plug |
Lap. repair |
Herniotomy |
Other |
|---|---|---|---|---|---|---|---|
N |
429 |
181 |
79 |
45 |
10 |
9 |
8 |
Complications |
|||||||
None |
355 |
144 |
69 |
33 |
5 |
8 |
7 |
82.8% |
79.6% |
87.3% |
73.3% |
50.0% |
77.8% |
87.5% |
|
Seroma |
12 |
15 |
0 |
1 |
1 |
0 |
1 |
2.8% |
8.3% |
0.0% |
2.2% |
10.0% |
0.0% |
12.5% |
|
Infection |
13 |
9 |
2 |
2 |
0 |
0 |
0 |
3.0% |
5.0% |
2.5% |
4.4% |
0.0% |
0.0% |
0.0% |
|
Sensory loss |
12 |
0 |
3 |
2 |
0 |
0 |
0 |
2.8% |
0.0% |
3.8% |
4.4% |
0.0% |
0.0% |
0.0% |
|
Pain |
29 |
12 |
5 |
7 |
2 |
0 |
0 |
6.8% |
6.6% |
6.3% |
15.6% |
20.0% |
0.0% |
0.0% |
|
Recurrence |
1 |
0 |
0 |
1 |
2 |
0 |
0 |
0.2% |
0.0% |
0.0% |
2.2% |
20.0% |
11.1% |
0.0% |
|
Other |
19 |
8 |
1 |
1 |
0 |
1 |
0 |
4.4% |
4.4% |
1.3% |
2.2% |
0.0% |
11.1% |
0.0% |
|
Reoperation |
3 |
2 |
1 |
0 |
0 |
1 |
0 |
| PHS=Prolene Hernia System Adapted from Anand A, et al. Indian J Surg. 2011 Table 2: Early Postoperative Complications (only patients who had 6–8 weeks follow up: n = 761) |
|||||||
Parameter |
Desarda (n = 96) |
Lichtenstein (n = 92) |
p* |
|---|---|---|---|
12-Month follow-upa |
|||
Foreign body sensation |
13 (14.6%) |
17 (18.1%) |
0.525 |
Abdominal wall stiffness |
14 (15.7%) |
20 (21.3%) |
0.335 |
Loss or change of sensation in the operated groin |
36 (40.4%) |
42 (44.7%) |
0.563 |
24-Month follow-up |
|||
Foreign body sensation |
14 (15.2%) |
16 (17.6%) |
0.666 |
Abdominal wall stiffness |
15 (16.3%) |
18 (19.8%) |
0.541 |
Loss or change of sensation in the operated groin |
38 (41.3%) |
41 (45.1%) |
0.609 |
36-Month follow-upc |
|||
Foreign body sensation |
10 (12.2%) |
16 (18.8%) |
0.238 |
Abdominal wall stiffness |
10 (12.2%) |
19 (22.3%) |
0.083 |
Loss or change of sensation in the operated groin |
36 (40.4%) |
40 (38.8%) |
0.386 |
| Adapted from JacekSzopinski, et al. World J Surg. (2012)
Table 3: Comparison of outcomes of Desarda Versus Lichtenstein operative methods |
|||






































