Top Links
Journal of Stem Cells and Clinical Practice
Mesenchymal Stem Cells in the Treatment of Patients with Chronic Spinal Cord Injury (ASIA A) with Residual Electrophysiological Function
Copyright: © 2015 Araujo AS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Introduction: Bone-marrow derived mesenchymal stem cells (MSC) represent an experimental form of therapy in the treatment of chronic spinal cord injury (SCI), up to two years after trauma. These cells may facilitate the functional recovery of SCI patients, as well as improve the radiological and electrophysiological aspects of this myelopathic disease. MSC therapy is still considered experimental, and is therefore limited to cases where no other suitable technique is available and where all involved individuals provide informed consent.
Methods: Ideal candidates for neuroregenerative treatment with MSCs are patients with cervical or thoracic spinal cord injuries who have a "discomplete clinical syndrome" (with electromyographic evidence of residual suprasegmental activity) and MRI showing syringomyelic cavitation at the level of spinal cord injury (without glial scars or other spinal cord damage or compression).
Case report: We present the first patient included in our protocol of MSC application for the treatment of chronic spinal cord injury with syringomyelic cavitation. Following 6 months follow-up, patient obtained remarkable radiological and electrophysiological improvements, as well as mild motor functional recovery. He recovered paravertebral muscle control and regained postural trunk stability.
Keywords: Mesenchymal stem cells; Traumatic syringomyelia; Spinal cord injury
In Brazil, the incidence of spinal cord injury (SCI) reaches 40 new cases/year/million. In other words, there are almost 6 to 8 thousand new cases per year, 80% of which are males and 60% falls between the ages of 10 and 30 [1].
The severity of SCI can vary from complete paraplegia to incomplete myelopathy or paraparesis. In addition to SCI severity, the type of trauma is a crucial factor determining the chances of recovery and the type of therapy selected. The initial trauma causes stretching and tearing of the nervous tissue, along with the interruption of axonal transmission. This is followed by axonal and neural degeneration, as well as glial scar formation [2].
In many cases of SCI, the treatment algorithm involves spinal decompression with realignment and mechanical stabilization, the prevention of ischemia and demyelination (caused by the inflammatory cascade), and promotion of neural regeneration.
The first report of the spine's regenerative capacity was published in 1980 [3], and since then, two forms of therapy have emerged: one that aims to prevent the inflammatory response (neuroprotection) and the other that tries to induce remyelination and axonal and neural regeneration (neuroregeneration).
The inflammatory cascade begins 1-2 days after trauma with granulocytic infiltration and 5-7 days after trauma with macrophage infiltration. At this stage, the macrophages facilitate phagocytosis of the cellular debris and myelin remains, and release trophic factors for endogenous ependymal NSCs in order to activate neuroregeneration. Cellular induction for ependymal NSC differentiation peaks at 7 days post trauma [2].
However, 3 weeks after trauma, these same factors lead to reactive astrocytosis, leading to glial scar formation, which in turn blocks neuroregeneration. In other words, exacerbated inflammation stimulates neuronal loss following SCI, while controlled inflammation stimulates neuroregeneration [2].
Activating and promoting endogenous NSCs are particularly important processes because they result in approximately 500,000 – 2,000,000 new cells at the site of the lesion one month following trauma [2]. Implanting stem cells can reactivate this process.
Recent advances in molecular biology have brought to light scientific, political and ethical discussions regarding the use of stem cells (SC). The regenerative capacity of stem cells from various tissues and their recently discovered role in the process of oncogenesis open up a whole new area of therapeutic possibilities.
One of the greatest challenges of modern biology is understanding how a complex multicellular system can develop from a single cell or line of cells [4]. The term 'stem cell' was first used in the 1960s when a limited number of transplanted bone marrow cells formed erythrocytic colonies in the spleens of irradiated mice [5,6].
To be classified as such, SC must be able to: a) self-multiply; b) produce all cell types present in a given tissue; and c) remain for a significant period of time in the host's life.
Following loco-regional stimuli, SCs differentiate into multipotent cells of the ectoderm, mesoderm and endoderm, which in turn become tissue stem cells (mesenchymal, skin, blood, etc.) or organ-specific cells, such as neural stem cells.
Neural stem cells (NSC) present a hierarchical organization that is similar to that of hematopoietic cells. As multipotent cells proliferate and self-renew, progenitor cells lose their ability to proliferate or differentiate into other lineages. In other words, younger cells have a lower capacity to differentiate.
NSCs can differentiate into lineages of neurons, oligodendrocytes or astrocytes. In vitro, NSCs are arranged in spherical formations known as 'neurospheres' [7]. Few neurosphere-derived cells can produce neurons or glial cells when transferred to rodents' brains. In the adult human brain, NSC-containing neurospheres have been isolated from the subventricular zone and the dentate gyrus of the hippocampus, by brain biopsy [8].
Uchida et al. were the first to use immunofluorescence staining of cell membrane proteins (p. ex. CD 133+, CD24neg/lo, CD45neg) from fetal subventricular zone [9]. When selected In vitro, these cells were able to replicate in culture and create 'neurospheres' capable of differentiating into neurons, astrocytes and oligodendrocytes. These cells also have the ability to engraft, migrate and differentiate when transplanted into the brains of immunocompromised newborn mice [10].
When they were injected into mice with traumatic spinal cord injuries, these cells were also able to improve motor function [11]. Although the mechanism underlying this improvement is not entirely clear, it is thought to involve the differentiation of NSCs into myelinated oligodendrocytes on a mesh of astrocytic lineage [12,13].
Applying a heterologous NSC graft in an animal research model requires the simultaneous application of corticosteroids and long periods of host immunosuppression. Therefore, a new technique emerged that uses endogenous stem cells derived from bone marrow; these cells are mostly mesenchymal stem cells (MSCs). These cells may also be obtained from adipose tissue or umbilical cord blood.
Until recently, there were few stem cell studies conducted in humans, which is due mostly to the ethical questions associated with the use of embryonic stem cells. However, the advent of endogenous stem cells derived from bone marrow is changing this scenario quite dramatically.
The low regeneration potential of the central nervous system (CNS) represents a challenge for the development of new therapeutic strategies. Mesenchymal stem cells (MSCs) have been proposed as a possible therapeutic tool for CNS disorders [14].
In vitro experiments have demonstrated that neuronal-like cells derived from bone marrow MSC can survive, migrate, integrate and help to restore the function and behaviors of SCI models, and that they may serve as a suitable approach to treating SCI [15-18]. However, it is very difficult to track transplanted cells in vivo. Zhang et al. first proved that transplanted superparamagnetic iron oxide-labeled neuronal-like cells derived from bone marrow MSCs can migrate into the rabbit model SCI region and can be tracked by magnetic resonance in vivo [19].
In experimental animal model studies of SCI, MSC injections resulted in significant tissue preservation, a reduction in syringomyelic cavitation and glial scarring, and improved motor function [15-17].
In a long-term prospective clinical study, Park et al. (2012) reported improved radiological, electrophysiological and functional motor profiles in 3 out of 10 patients with complete cervical lesions, who received intramedullary and intradural injections of bone marrow-derived MSCs [20]. Unfortunately the motor recovery was observed only in segments closely adjacent to the spinal cord lesion.
In Park et al. study, 10 patients with severe spinal cord injury underwent bone marrow puncture of the iliac bone to culture MSCs for a period of 4 weeks [20]. Next, they underwent a laminectomy of the syringomyelic cavity that involved opening the dura mater and applying an intramedullary injection of 1ml saline solution containing 8x106 MSCs, and an intradural injection of 5ml with 4x107 MSCs. Then, at 4 and 8 weeks, the patients received a lumbar puncture with an intrathecal injection of another 8ml of saline solution containing 5x107 MSCs.
Around the 6-month follow-up, 6 of the 10 patients showed improved motor strength, and 3 of them presented a gradual improvement in their daily activities. These same 3 patients also showed a reduced syringomyelic cavity, an increase in the density and diameter of the spinal cord, as well as improved motor and somatosensory evoked potentials [20]. Throughout the study, they did not observe any adverse events related to the application of MSCs or any malignant degeneration (teratoma), as has been previously reported for treatments with embryonic stem cells.
After multiple trauma patients receive the appropriate primary care, those suspected of having a spinal cord injury must be evaluated by a neurosurgeon in order to identify the etiology of the lesion, the level(s) where injury occurred and their severity, the degree of sensorimotor deficits, and subsequent exams that must be ordered to further evaluate the patients' condition.
Immediately following the relevant exams, the SCI must be categorized according to the ASIA (American Spinal Injury Association)/ ISCoS (International Spinal Cord Society) scale (which is recognized and used worldwide), and to the SCI etiology, in order to assemble treatment options, such as surgery or immobilization/traction [21,22].
The ASIA classification contains the following items: a) assessment of the sensorimotor level of injury based on the conscious somatosensory perception test and the motor control test; b) determining whether the lesion is complete or incomplete based on the complete loss or preservation of sacral sensorimotor function; c) determining the severity of the lesion (ASIA score A, B, C, D or E); and d) estimating the region of partial sensorimotor preservation in patients with complete lesions [21].
In 1976, Guttmann was the first to describe spinal cord lesions as being clinically complete or incomplete [23]. A clinically complete lesion results from the total transverse cut of the spinal cord, resulting in the complete loss of voluntary sensory and motor function below the level of the lesion [23]. On the other hand, a clinically incomplete lesion arises from a partial lesion of the spinal cord, resulting in a partial preservation of sensorimotor functions below the level of the lesion. Incomplete lesions may be divided into two groups: a) a diffuse lesion affecting an entire spinal segment, with damage to the central gray matter and to the long ascending and descending white matter tracts, even with low intensity; and b) an anatomically circumscribed lesion of different parts of the spinal cord, with dissociated deficits linked to the site of damage, e.g. anterior cord syndrome, central cord syndrome (Schneider), or posterior or medullary cone syndrome.
Since the advent of modern neurophysiological assessments, some patients who were previously thought to have complete spinal cord lesions have been shown to have remaining supraspinal signals that influence their somatosensory potentials by traversing the site of spinal cord damage.
For example, a noticeable increase in the amplitude of the potential was observed when these patients underwent polyelectromyographic skin monitoring and performed the Jendrassik maneuver or a voluntary neck flexion.
Autopsy studies confirm this result, revealing the preservation of a small number of axons crossing the site of the lesion that could be responding to suprasegmental input [24].
This subclinically incomplete (yet clinically complete) syndrome in which suprasegmental input can affect deep spinal reflexes or even the somatosensory evoked potentials below the level of the spinal injury was called "discomplete clinical syndrome" (DCS) by Kakulas, Dimitrijevik et al. [25].
Because of this residual (even if subclinical) suprasegmental control, these patients are ideal candidates for neuroregenerative treatment of the spinal cord. Theoretically, the SCs would only amplify an existing ¨neural circuitry¨ background activity in patients with DCS, instead of creating new circuitries in patients with electrophysiologically complete SCI. Perhaps this residual background activity would represent the ¨input¨ necessary to the MSCs to differentiate into neurons.
Ideal candidates for neuroregenerative treatment with MSCs are patients with cervical or thoracic spinal cord injuries who have a "discomplete clinical syndrome" (with electromyographic evidence of residual suprasegmental activity) and MRI showing syringomyelic cavitation at the level of spinal cord injury (without glial scars or other spinal cord damage or compression). Unfortunately, in order to guarantee anatomical viability to intramedullary MSC infusion (syringomyelia), most SCI patients would not be eligible to this treatment.
Some confounding factors (such as the presence of SCI with incomplete clinical syndrome and other types of spinal cord damage) served as exclusion criteria in our study, since in these cases, it is impossible to attribute sensorimotor improvement exclusively to the neuroregenerative treatment. Patients with incomplete lesions could display sensorimotor improvement thanks to physical therapy or even to the natural progression of the disease, as has been observed in patients with central cord lesions. Patients with other spinal compressions could be benefiting from a specific surgical treatment, thus distorting the effects of the neuroregenerative treatment.
Furthermore, patients with complete spinal cord lesions who meet all the clinical and neurophysiological criteria (i.e., complete loss of motor control and sensibility below the level of the lesion), with electromyographic silence (without supraspinal signals or conscious influence on spinal reflexes), should also be excluded from neuroregenerative treatments with MSCs.
A 47-year-old male ophthalmologist experienced a sudden onset of intense right lumbosciatalgia, followed by anesthetic paraplegia. He was admitted to the hospital 10 hours after symptom onset, maintaining flaccid paraplegia with dense sensory loss at T4 and absent bulbocavernous reflex.
The patient underwent an MRI of the spine, which revealed extensive subarachnoid hemorrhage spanning from C7 to S1, with compressive myelopathy at D4-D6 and multisegmental myelomalacia (Figure 1). On the lumbar MR scan, we also observed tethered cord with low-placed conus at L4 and S1-S2 sacral lipoma with flow void of the lumbar epidural vessels (indicative of lumbosacral arteriovenous malformation).
The patient was immediately submitted to a D4-D6 laminectomy with durotomy and subarachnoid hematoma drainage using a craniocaudal urethral probe. The next day, the patient underwent spinal angiography with embolization of the extensive lumbosacral AVM fed by terminal lumbar branches.
Despite the surgical and endovascular treatment, the patient had neurological sequelae with mild postoperative improvement, but no motor improvement (ASIA A), and was discharged with a recommendation for physical therapy.
At follow-up, the patient had developed an arachnoid cyst at D8 and a syringomyelic cavity at D10, both of which compressed the residual spinal cord. These were treated one year ago with the fenestration of the arachnoid cyst at D8 and a syringosubarachnoid shunt with insertion of a T-tube at D10 (Edwards-Barbaro Syringo-Peritoneal Shunt – Integra Neurosciences) – (Figure 2).
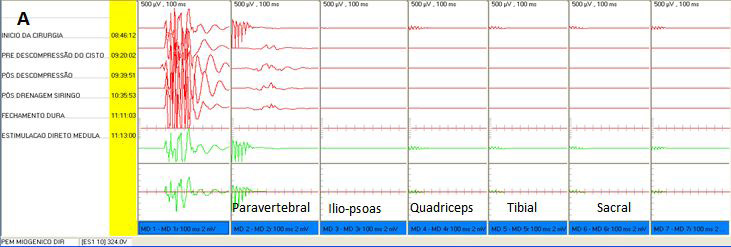
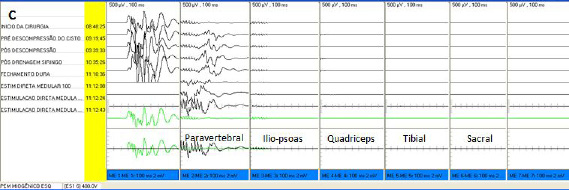
During and immediately following surgery, the patient underwent monitoring of somatosensory and motor evoked potentials (Figure 5A and C), which revealed very low paravertebral activity and no activity below the iliopsoas.
Given that our patient did not show any sensorimotor improvements in the year following the surgery to drain the arachnoid cyst and the syringomyelic cavity, we opted for the use of MSCs with the goal of inducing neuroregeneration of the damaged spinal cord and resolving the syringomyelic cavity. Once the patient and his family had given their consent, and our hospital's Clinical Research Ethics committee approved the method, we submitted the patient to the protocol of MSC infusion described by Park et al. [20] (Figure 3).
Approximately 200 ml of bone marrow were extracted from the patient's iliac crest following the protocol described by Thomas et al. (1975).
The patient agreed to participate in the study and signed an informed consent form. After collection, the material was submitted to separation of mononuclear cells by Ficoll-PaqueTM PLUS (GE Healthcare, Little Chalfont, Buckinghamshire, UK), as described by Ahmadbeigi et al. (2012), and immediately washed with physiological solution and centrifuged at 500 g for 5 minutes.
The mononuclear cells (~106) were plated in 75 cm2 culture flasks (Corning Inc., Corning, NY, USA) and cultivated according to Lizier et al. (2012) in Dulbecco's Modified Eagle's Medium (DMEM) /Ham's F12 (DMEM/F12, Invitrogen Corporation - Carlsbad, CA, USA) supplemented with 15% fetal bovine serum (FBS, Hyclone, Logan, Utah, USA), 100 units/ml of penicillin, 100 μg/ml of streptomycin, 2 mM of L-glutamine, and 2 mM of non-essential amino acids (all from Invitrogen) in relative humidity at 37 °C and 5% CO2 (incubator - Thermo Fisher Scientific Inc, Waltham, MA, USA). After 3 to 7 days, fibroblast cells began to appear in a monolayer. These were washed twice with phosphate-buffered solution (PBS - Invitrogen, Carlsbad, CA, USA) and subjected to enzymatic dissociation with TrypLE (Invitrogen) for 3 to 6 minutes at 37 °C.
The first step was counted after the first enzymatic dissociation. The effect of TrypLE was inactivated by culture medium with 10% FBS and the cells (~5×105) were plated in 75 cm2 culture flasks (Corning). This subculture protocol was carried out every 7 to 14 days and the culture medium was replaced every 3 to 4 days. We prepared a medium for cryopreservation with 90% plasmin (Halex Istar, Goiania, GO, Brazil) and 10% Dimethyl Sulfoxide (DMSO) (OriGen Biomedical, Austin, TX, USA). The cryopreserved cells were kept in toggles (Corning) at -196 °C.
Immunophenotyping was based on flow cytometry using FITC conjugated antibodies specific to human proteins, such as CD90 (mesenchymal marker) and CD34 (hematopoietic marker) and their respective control isotopes (all from BD Pharmingen, Franklin Lakes, NJ, USA).
The cells in step three were dissociated for enzymatic treatment (6 min at 37 °C with TrypLE), centrifuged (10 min at 400 g) and washed with PBS at 4 °C. Next, at a concentration of 105 cells/ml, the cells were incubated with the antibodies mentioned above (1 μl). After 45 minutes of incubation protected from light and at room temperature, the cells were washed three times with PBS and resuspended in 0.25 ml of cold PBS. The analysis was conducted using a fluorescence-activated cell sorter (FACSCanto II; Becton, Dickinson, San Jose, CA) and the CELL Quest program (Becton, Dickinson).
For this procedure, we extracted approximately 2 ml of conditioned medium from the flasks of mesenchymal stem cell culture and then added them to the microbiological growth bottle (BD Bactec Peds PlusTM). This control detects the growth of aerobic and anaerobic microorganisms in medium samples after approximately five days, as they contain antibiotic-inhibiting resins (PLUS media).
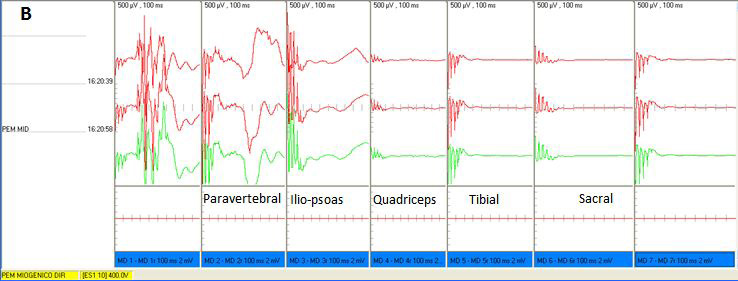
Three months after the last application of MSCs, we conducted a radiological assessment with MR of the thoracolumbar spine (Figure 4) and an electrophysiological assessment with motor evoked potentials (Figure 5B and D).
Despite the presence of significant multisegmental adhesive arachnoiditis (which can limit the assessment of spinal cord diameter and thickness), an improvement in the syringomyelic cavity at D10 was visible on the thoracolumbar scan (Figure 5).
Our patient is still being followed, we are now up to one year after the last MSC infusion, and we could testify astonishing improvements.
In neurological evaluation, before MSC treatment, patient had complete tactile and thermo-algesic anesthesia and abolition of voluntary motion below D4 level (ASIA A). Despite it, electrophysiological monitoring revealed residual suprasegmental electrical activity passing through D4 level (when patient was asked to perform Jendrassik maneuver and neck flexion a tiny change in amplitude waves of paravertebral muscle was observed in polyelectromyographic skin monitoring). Thus this patient was diagnosed as harboring a ¨Discomplete clinical spinal cord syndrome¨ (DCS), and the bone marrow-derived MSCs Park`s protocol was then applied [20].
In further electrophysiological and clinical assessment, we observed an improvement in the motor potentials of both inferior limbs, with apparent normality in the bilateral paravertebral segment (which allowed our patient to maintain a seated posture with perfect equilibrium of the trunk, thus allowing him to return to his surgical work), iliopsoas improvement (he is currently able to flex the trunk in a satisfactory manner), improvement in the quadriceps (with regained tone and bilateral thigh tropism), and absence of function below the quadriceps. Nowadays patient is able to stand alone with the aid of lower-limb orthesis and canes (Figure 6).
Another relevant topic following the MSC transplantation was the troublesome neuropathic and myelopathic pain that affected patient since 3 months after the hemorrhage onset. This pain was so intense that let him dependent of opioid medications (oral Methadone and transcutaneous Fentanyl). Noteworthy was that patient has became medication-free after the MSC treatment, without any pain. This may be explained by the immunomodulatory efficacy of the intrathecal MSC therapy [29].
The application of MSC over embryonic stem cells is largely justified, since it is easier to obtain, to harvest, and to storage, with lower cost, and without ethical implications. Furthermore up to now there is no report of tumorigenesis using MSC transplantation. We use bone marrow-derived cells since it is easy to obtain, but adipose-derived or umbilical cord blood-derived cells may be an option.
The selection of patients eligible to MSCs therapy is still an issue of debate. This is the first report that applies MSCs therapy in DCS patients. Perhaps the MSC therapy in paraplegic anesthetics patients (ASIA A) with residual electrophysiological function may amplify a minimal electrical activity passing through the lesion level.
Theoretically, the SCs would only amplify an existing ¨neural circuitry¨ background activity in patients with DCS, instead of creating new circuitries in patients with electrophysiologically complete SCI. Perhaps this residual background activity would represent the ¨input¨ necessary to the MSCs to differentiate into neurons.
Although these are only the preliminary results from the first patient included in our MSC protocol for SCI patients, the clinical, radiological and electrophysiological improvements presented by our patient suggest that this form of therapy is promising. Further studies will provide additional data and allow us to make stronger conclusions.
 |
| Figure 1: Preoperative spine MRI. (A) Sagittal lumbar T2 image showing extensive subarachnoid hemorrhage with lumbosacral flow void and S1-S2 lipoma; (B) T2 Axial view of large hematoma with significant compression of the lumbar dural sac; (C) T2 sagittal view of thoracic extension of the hematoma, with significant spinal cord compression at D4-D6; (D) Angiography of lumbosacral AVM responsible for hemorrhage prior to embolization. |
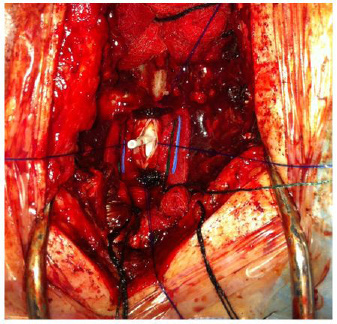 |
| Figure 2: Syringosubarachnoid shunt with insertion of a T-tube |
 |
| Figure 3: Illustration of the spinal cord at the level of the syringomyelic cavity and the subarachnoid space. (A) First infusion of mesenchymal stem cells under microsurgical visualization, 1ml (8x106 cells) divided into 5 sections of the syringomyelic cavity, and another 5 ml (4x107 cells) before dural closure of the subarachnoid space; (B) Second infusion after 4 weeks and third infusion after 8 weeks, with lumbar puncture for the infusion of 8 ml (5x107 cells) into the subarachnoid space. (Method based on Park et al) [20]. |
 |
| Figure 4: (A-B) T2 sagittal view of the thoracic spine, showing significant multisegmental adhesive arachnoiditis with syringomyelia at D10, which was operated on and submitted to intracavitary infusion of mesenchymal stem cells; (C-D) T2 axial view of the thoracic spine showing arachnoiditis with anterior and posterior adhesions |
 |
 |
 |
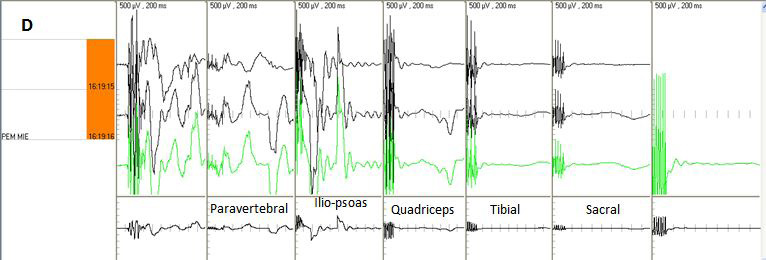 |
| Figure 5: Motor evoked potential of right lower limb with 400V precentral gyrus stimulation. (A) Pre MSC infusion, showing minimal residual paravertebral potential and isoelectric silence below the iliopsoas segment; (B) Post MSC infusion, showing significant improvement of wave amplitudes of the paravertebral, iliopsoas, quadriceps, tibial and sacral potentials; paravertebral segment potentials were normal and iliopsoas segment potentials were near normal following treatment. Motor evoked potential of the left inferior limb with 400V stimulation. (C) Pre MSC infusion, showing mild residual paravertebral activity and isoelectric silence below the iliopsoas segment; (D) Improved potential amplitude, with near normal amplitude in the paravertebral and iliopsoas segments and subnormal amplitude starting at the quadriceps |
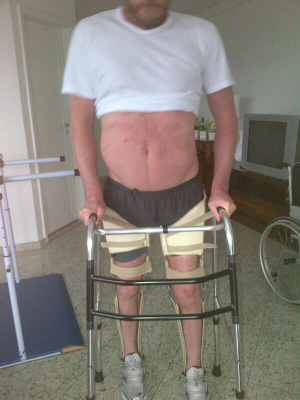 |
| Figure 6: Patient is now able to stand straight with the aids of orthesis |






































