Top Links
Journal of Gynecology Research
ISSN: 2454-3284
Effects of Chlorhexidine on Preventing Puerperal Infection and Neonatal Outcomes: A Meta Analysis
Copyright: © 2023 Yuhua Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Puerperal infection is a public health threat which may lead to maternal death and poor neonatal outcomes. Chlorhexidine is a representative antiseptic that is effective against the bacteria colonized in the reproductive tract of women. But researcher’s attitude towards the antimicrobial effects of chlorhexidine on pregnant women are conflicting. We sought to evaluate the preventive effect of chlorhexidine on puerperal infection and neonatal outcomes.
To identify the study we needed, Eight databases were searched for articles published in any language from January 1990 to December 2019. Randomised controlled trials that investigated the effect of chlorhexidine on preventing puerperal infections and neonatal outcomes related to the infection were included. Publications were assessed for inclusion. Relative risks from individual studies were pooled using a fixed model or random effects model. The heterogeneity of intervention was assessed by Chi2 and I2 tests. Nine randomised controlled trials evaluated the consequences of chlorhexidine interventions in postpartum infection. Our meta-analysis found that chlorhexidine can significantly reduce the prevalence of maternal fever (OR = 0.54, 95% CI 0.32-0.91) and neonatal death (OR = 0.38, CI 0.19-0.77). However, there is no significant differences between the intervention and control groups for puerperal infection (OR = 0.82, 95% CI 0.55 -1.20) and neonatal sepsis (OR = 0.83, CI 0.51-1.36). Our meta-analysis indicates that chlorhexidine has a preventive effect on postpartum fever and neonatal death.
Keywords: Puerperal infection; Postpartum infection; Chlorhexidine; Prevention; Systematic review
Puerperal infection is a systemic or local infection that occurs when the birth canal is invaded by endogenous or exogenous pathogenic bacteria during delivery or puerperium. Despite that the incidence of puerperal infections has declined significantly in recent years which is attributable to the advancement of childbirth technology and the application of antibacterial drugs, puerperal infection is still the sixth-leading cause of maternal death around the world. The major pathogens of puerperal infections include Group B streptococci (GBS), Enterococcus faecalis and Streptococcus aureus, etc [1] Once the puerperal infection occurs, it will seriously affect the postpartum recovery of the puerpera and the condition of newborn. Most of the puerperal infections may be secondary to some primary diseases discussed above. In such cases, the immune system of mothers is susceptible to invasion by pathogens. And infants that are exposed to those microorganisms colonized in the reproductive tract have a great chance to develop into neonatal sepsis.
The major way of delivery in the clinic includes vaginal delivery and cesarean section. Different delivery methods have different effects on the health of mothers and infants. Vaginal delivery is an internationally recognized healthy delivery method advocated by the World Health Organization (WHO). However, people’s perceptions about birth have altered within the past decades. The rate of cesarean section has been increasing, and the proportion of vaginal delivery has decreased significantly [2,3] The rate of cesarean section in the United States rose from 20% to over 30%, while it increased from about 20% to 46. 2% in China, far exceeding the requirement of the WHO to control the cesarean rate within 15% [4].
The current approach to reduce the risk of infection after delivery is antibiotics. Despite the broad-spectrum antibacterial activity of antibiotics [5], recent researches have validated that antibiotic prophylaxis did not reduce the morbidity of puerperal infection and neonatal sepsis [6,7]. The pathogens of puerperal infections are mainly anaerobic bacilli and streptococci. Conventional antibiotics (such as penicillin) are not ideal for treatment, and it is difficult to achieve the purpose of controlling inflammation. Therefore, other strategies like vaginal irrigation with antiseptics have been attempted by obstetricians to forestall the postpartum infection and the vertical transmission of microbes [8].
Chlorhexidine (CHX) is a representative cationic surfactant antiseptic [9] that is effective against most gram-positive and gramnegative bacteria including the bacteria colonized in the reproductive tract of women [10]. It exhibits the bactericidal effect by destroying the the integrity of bacterial cell wall [11]. But researcher’s attitude towards the antimicrobial effects of chlorhexidine on pregnant women are conflicting. In 1995, Adriaanse reported that that vaginal cleansing by CHX could reduce the incidence of GBS sepsis [12]. However, after investigated the efficacy of CHX in 1997 and 2003, Rouse et al. contend that CHX vaginal irrigation lacked efficacy in the prevention of clinically diagnosed maternal and neonatal infectious morbidity [13,14].
Analyzing the chlorhexidine and its efficiency can help improve the prevention of maternal and neonatal outcomes. To testify the role of chlorhexidine in preventing puerperal infections, we conducted a meta-analysis and demonstrate that CHX may not reduce the incidence of puerperal infection and neonatal sepsis.
To identify the study we needed, we searched articles on PubMed, Web of Science, Embase, Cochrane Central and Chinese National Knowledge Infrastructure from January 1990 to December 2019. We also adopted the search strategy of Medical Subject Heading (MeSH). The terms we used were: (Chlorhexidine) and (vaginal) and ((puerperal infection) or (postpartum infection) or (intraamniotic infection) or (endometritis) or (sepsis) or (vaginal irrigation) or (vaginal cleansing) or (vaginal douche) or (vaginal wipes) or (antiseptic) or (labor) or (pregnancy) or (maternal) or (neonatal)).
Only randomized controlled trials (RCTs) that evaluated the effect of chlorhexidine on puerperal infection and infection-related neonatal outcomes were included in this study. The intervention can be vaginal irrigation, wipes or any other form of chlorhexidine. Participants in control group might have received vaginal cleansing with normal saline or water. Case reports, reviews or letters were excluded from final analysis.
Author Pingping Zhang searched the articles alone. To refine the data collected from the different databases, two authors (Jingjing Bi, Yuchun Song) read the abstracts or the methods section to evaluate the article is qualified. Finally, Haining Wang pored over each paper and decided whether the article should be included. Data was extracted by two independent researchers (Jingjing Bi, Pingping Zhang) and then transferred to Review Manager Software Revman 5.3 (Cochrane, USA).
Outcomes measures including (1) puerperal infection morbidity, which can be the combined morbidity of intraamniotic infection and endometritis (2) postpartum fever, which the body temperature was greater than 38 ℃ (3) neonatal sepsis (4) neonatal death related to the infection.
Two authors (Jingjing Bi, Pingping Zhang) independently assessed risk of bias for each study based on method described in Cochrane Handbook for Systematic Reviews of Interventions. Any disagreement was resolved by discussion or by involving a third assessor.
Data are expressed as Relative risk (RR) or Odds ratio (OR) and its 95% confidence interval (CI). For the heterogeneity test included in the study, I2 test and Chi2 test were used, and the test level was α = 0.05. If there was no statistical heterogeneity between the studies (P> 0.1, I2 < 50%), a fixed effect model was used for analysis. Otherwise, a random effect model was used for analysis. Publications bias was evaluated by the Begg test or Egger test.
We searched articles from different database and performed meta-analysis regarding the effect of chlorhexidine on puerperal infection and neonatal outcomes. Odds radio and heterogeneity, publication bias and risk of bias were evaluated.
After preliminary searching and screening for papers that meet the needs of our research, a total of 32 related literatures were obtained as shown in Figure 1. No papers in Chinese or other languages were found. By reading the title, abstract, and content of the literature, articles that do not meet the inclusion criteria were excluded. Finally, nine RCTs [12-20] containing a total of 11018 mothers and 19020 infants were included. Among them, 5564 patients and 9740 infants were in the intervention group (patients treated with Chlorhexidine during labor) and 5454 patients and 9280 infants were in the control group. The basic information of the included studies is shown in Table 1.
As shown in Figure 2, six studies [13-16,19,20] (Figure 2) including 9265 participants (4619 in the intervention group and 4646 in the control group) have shown that there was no significant difference between the two groups in puerperal infection morbidity (OR=0.82, 95% CI 0.55 -1.20).
Four studies [12,15,17,18] revealed that 23 of 1047 patients in intervention group and 42 of 906 patients in the control group have a postpartum fever. The number of febrile patients was significantly reduced with chlorhexidine intervention (OR = 0.54, 95% CI 0.32-0.91, I2= 0 %, P = 0.96).
Four studies [13,14,16,18] (Figure 3) have recorded the morbidity of neonatal sepsis. A totally number of 9109 infants were included which 4529 of them were from the intervention group and 4580 of them were from the control group. There was no statistical heterogeneity among the four studies (P = 0.15, I2= 43%). A fixed-effects model was used for analysis. The results (Figure 4) have shown that there is no significant defferences in the morbidity of neonatal sepsis between the intervention group and the control group (OR = 0.83, CI 0.51-1.36).
Neonatal mortality related to infection was also evaluated (Figure 5). The number of foetal death in the intervention group is significantly lower than the control group (OR = 0.38, CI 0.19-0.77) as shown in Figure 5. No statistical heterogeneity was found between the two groups (P = 0.40, I2= 0), so a fixed effect model was used for analysis.
The present meta-analysis included a relatively small number of studies, making difficult to assess the publication bias. We performed Begg test and Egger test using Stata software. No significant publication bias was identified for puerperal infection (Begg test, P = 0.302), postpartum fever (Egger test, P = 0.314) and neonatal sepsis (Egger test, P = 0.828).
According to the 'Risk of bias' tool, one study (Taha 1997) had unclear risk of selection bias because of the difficulty of allocation concealment. One study (Ahmed 2016) has high risk of performance bias due to the presence of surgeon while the vaginal scrub was performed, and unclear risk of selection bias. Each risk of bias is summarised in Figure 6 and Figure 7.
In recent years, with the continuous discovery of new antibiotics, maternal deaths caused by puerperal infections have been significantly reduced. Nevertheless, due to the emergence of bacterial resistant strains, new difficulties have been added in treatment. To date, puerperal infections remain one of the leading causes of maternal mortality. Pathogens colonized in the vagina of pregnant women can not only cause puerperal infections, but also indirectly infect fetuses through placenta, membranes, and amniotic fluid during pregnancy, and cause miscarriages, preterm births, stillbirths and premature rupture of membranes. In addition, the contact with visitors during postpartum hospitalization is an extremely important factor for puerperal infections which is frequently neglected. Antepartum prevention of puerperal infection is mainly to strengthen the relevant inspection during pregnancy and. Antibiotics or antiseptics prophylaxis before birth can also effectively prevent the occurrence of puerperal infection. The results of our analysis indicated that vaginal irrigation of CHX may not reduce the incidence of puerperal infection and neonatal sepsis. However, it may reduce the incidence of maternal fever and neonatal death.
There is clinical heterogeneity in the studies analyzed (Table 1). First, there are differences in the intervention method. Though most of the studies included were using vaginal cleansing or douching, one study conducted by Adriaanse et al washed the vaginal using a kind of gel which the main ingredients are chlorhexidine, digluconate and glycerol. This procedure will be repeated 10 h after the first washing if the delivery was not initiated. Another study conducted by Cutland et al. used vaginal wipes instead of cleansing by syringes. Besides, apart from maternal vaginal douching, one study in our analysis also cleansed the body of infants. Second, the composition of solution used in control group is different. Some researchers douched the vagina using the sterile saline, but others just used the sterile water. This divergence may lead to a confounding result. Some studies included in their analysis the outcomes of mothers who underwent emergency caesarean section. Cesarean section can change the internal ecological environment of the female reproductive tract. Under normal circumstances, women's vagina contains a large number of anaerobic bacteria, these anaerobic bacteria can maintain a relatively stable number of bacteria. However, after a cesarean section, the number of anaerobic bacteria in the vagina can be significantly reduced and exogenous microorganisms can be increased at the same time. Cesarean section surgery can temporarily reduce the body's immune function, which significantly reduces its resistance. Decreased maternal immune function can significantly increase the number of pathogenic bacteria in the vagina and enhance the bacterial toxicity. Low maternal immune function can cause synergistic effects of conditional pathogens such as E. coli and Gardnerella to multiply rapidly in damaged mucosa, parauterine tissues and uterus. Therefore, the future study needs to separately count the number of puerperal infections in pregnant women who underwent caesarean section and vaginal delivery.
The number of infection-related neonatal death is around 626,000, which accounts for nearly one-fifth to one-third of the total neonatal deaths [21-24]. Most of the death were from low and middle-income countries [25]. Despite a decline in the incidence of puerperium infections in Europe, the United States still has a considerable mortality rate maternal death, with approximately 10% resulting from puerperal infection [26,27]. All the studies were conducted in developed countries in our meta-analysis except for Malawi, South Africa and Saudi Arabia. The outcome measures and conclusions seem to be inconsistent. In developed countries, Rouse et al’s contend that CHX may not prevent mothers from being infected. The rest of the studies declared that CHX have a preventive effect on mother and infants. Two the studies in underdeveloped countries considered that it has a promising effect on decreasing the rate of puerperal infections. The results of some non-randomized controlled clinical trials support the conclusions in the two studies [28]. However, one study from South Africa claimed that we need to find other effective antiseptics instead of using CHX [16].
Although there is controversy about whether chlorhexidine can prevent puerperal infections, most articles support that CHX can reduce the incidence of neonatal death. A previous review has assessed maternal and neonatal colonization and infectious morbidity and suggested that intervention may lead to an improvement in neonatal outcomes related to vertical transmission [29] and that was consistent with the results of our study
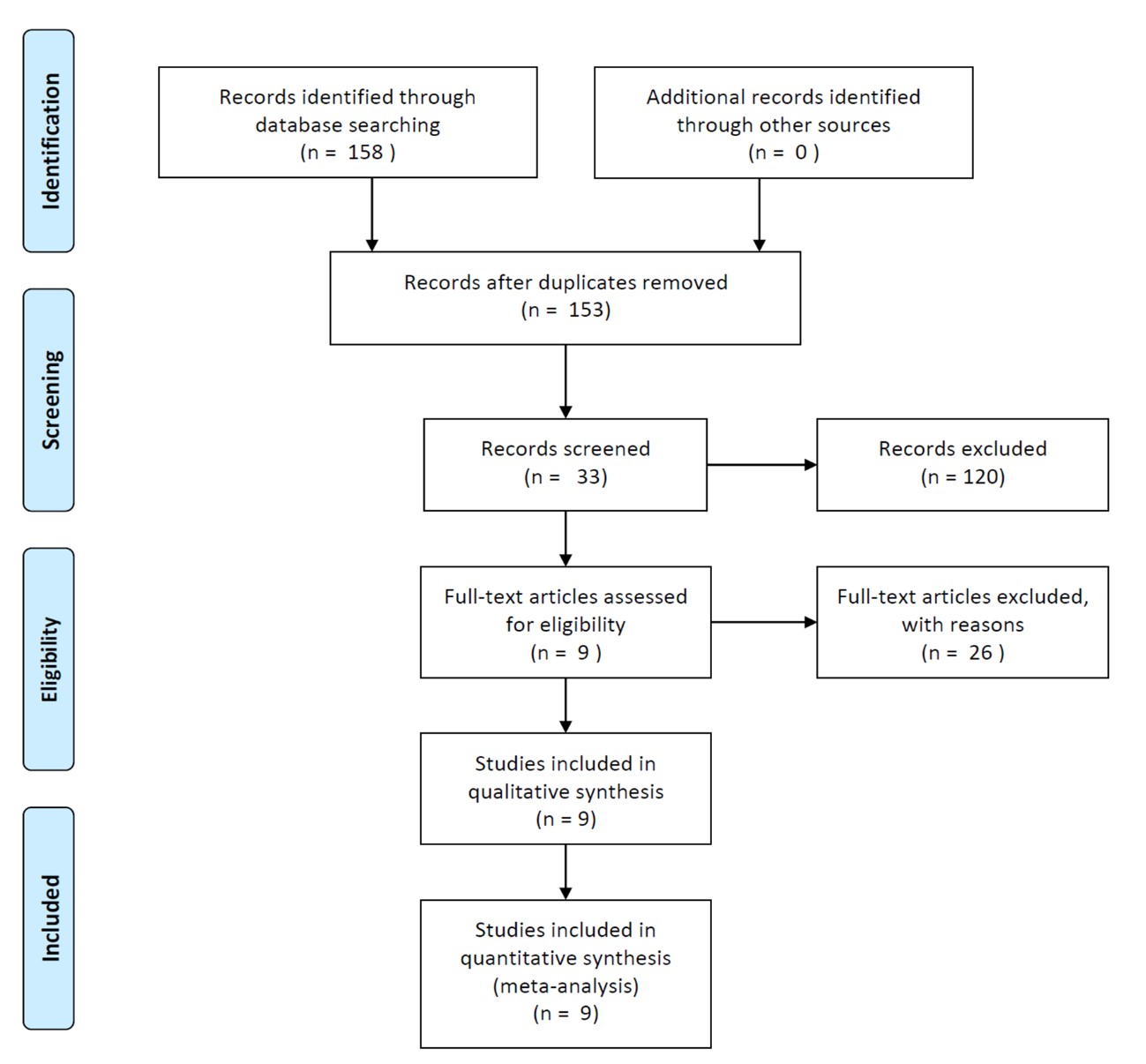 |
| Figure 1: PRISMA flow chart of search strategy |
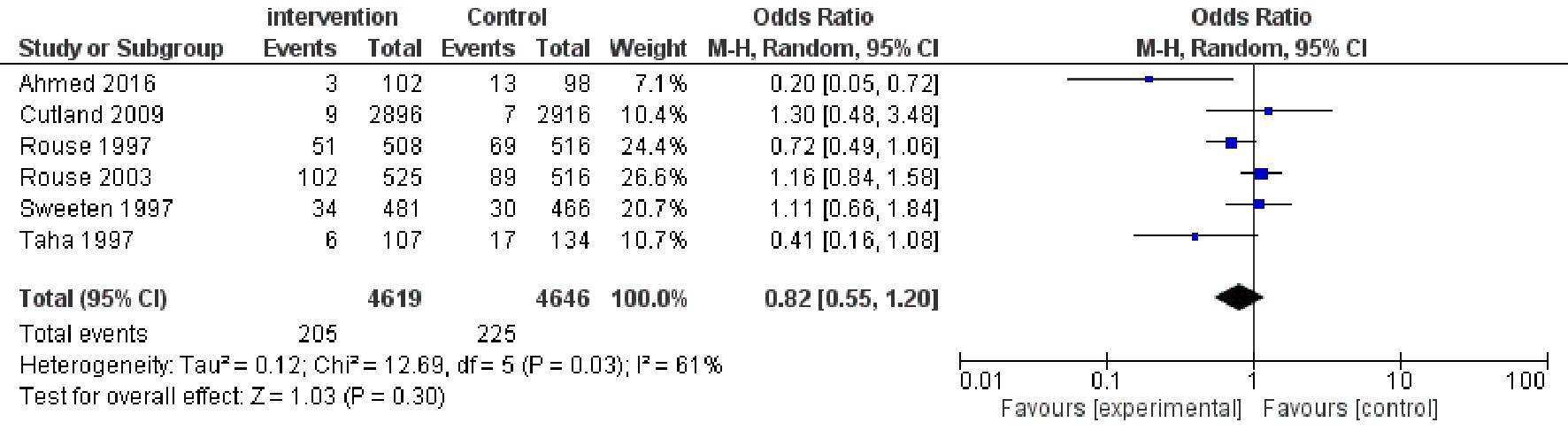 |
| Figure 2: Forest plot comparing the puerperal infection morbidity |
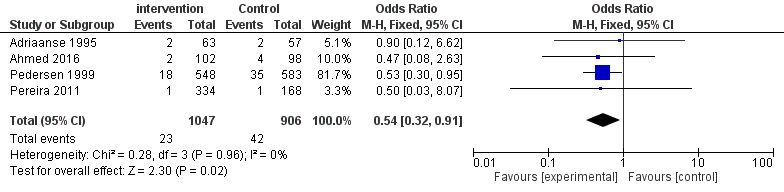 |
| Figure 3: Forest plot comparing the febrile morbidity |
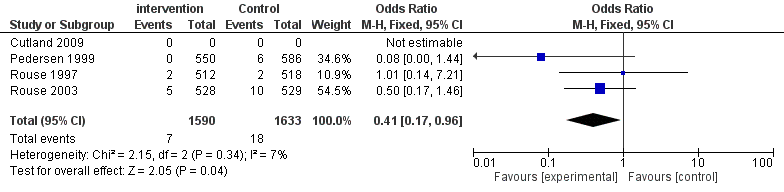 |
| Figure 4: Forest plot comparing the neonatal sepsis morbidity |
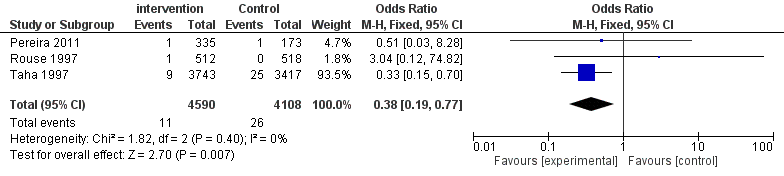 |
| Figure 5: Forest plot comparing the neonatal death |
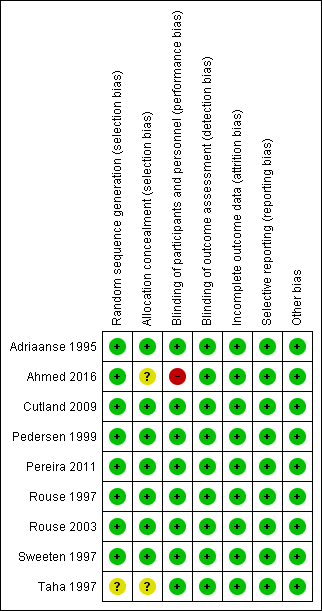 |
| Figure 6: 'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. |
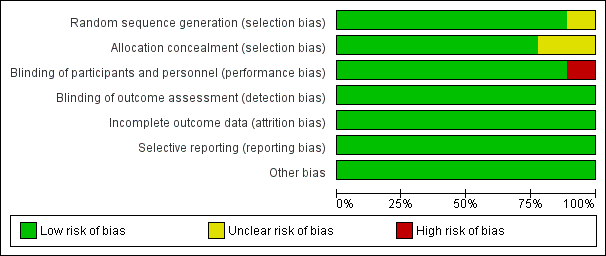 |
| Figure 7: 'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study |






































