Top Links
Journal of Genetic Mutation Disorders
Mthfr c677t, Homocysteine and Risk of Splanchnic Vein Thrombosis: A Pooled Analysis of Published Epidemiological Studies
Copyright: © 2025 Li SL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Objective: This meta-analysis aimed to comprehensively assess the literature examining a possible link between the methylenetetrahydrofolate reductase (MTHFR) C677T gene mutation and homocysteine and the risk of splanchnic vein thrombosis (SVT).
Methods: Electronic databases were searched up to November 2014 for published case- control studies investigating genetic polymorphisms or the homocysteine level associated with SVT patients, including Budd-Chiari syndrome (BCS), portal vein thrombosis (PVT) and mesenteric vein thrombosis (MVT). Data on year of publication, study design, country of origin, number of patients/control subjects, were abstracted. Pooled odds ratios (OR) or weighted mean difference with 95% confidence interval were calculated using a random-effects model.
Results: We initially identified and screened 72 relevant studies for retrieval, and included 24 in our final analysis. Statistically significant associations with SVT were found for MTHFR C677T (OR=2.09; 95% CI, 1.66 to 2.64; P<0.01). In subgroup analysis, similar results were also observed in PVT (OR=1.73, 95% CI=1.21 to 2.48, P<0.01) and MVT (OR= 3.14; 95% CI, 1.97 to 5.01, P<0.01). However, the meta-analysis of the OR found in our current study for MTHFR/C677T in BCS generated a pooled observed OR of 1.36 (OR=1.36; 95% CI, 0.61 to 3.02, P>0.05). The plasma homocysteine level was significantly higher in SVT patients than in healthy controls (SMD = 0.50, 95% CI = 0.12–0.87, P = 0.010)
Conclusion: Our results suggest that MTHFRC677T gene mutation and the homocysteine level may be associated with SVT.
Keywords: Splanchnic vein thrombosis; Budd-Chiari syndrome; Portal vein thrombosis; Mesenteric vein thrombosis; Methylenetetrahydrofolate reductase; Homocysteine; Meta-analysis
Splanchnic vein thrombosis (SVT), that is hepatic, portal, mesenteric or splenic vein thrombosis, is a rare and life-threatening vascular disorder associated with highly variable clinical expression, including Budd-Chiari syndrome (BCS), portal vein thrombosis (PVT) and mesenteric vein thrombosis (MVT) [1]. BCS results from hepatic venous outflow obstruction at any level, from hepatic venules to the right atrium [2]. PVT refers to thrombosis within the extrahepatic portal vein, mesenteric vein and splenic vein. BCS and PVT are two major vascular disorders of the liver [3]. Acute mesenteric venous thrombosis (MVT) is an uncommon disorder with non-specific signs and symptoms. The diagnosis of which requires a high index of suspicion [4]. SVT is an under diagnosed disease, with heterogeneous clinical presentations and a non-negligible rate of incidental findings. Several risk factors have been identified, including inherited or acquired coagulation disorders (that is, factor V Leiden, factor II, and antiphospholipid syndrome) [5,6].
Furthermore, methylenetetrahydrofolate reductase (MTHFR) located in 1p36.3, which plays an important role in processing amino acids and building blocks of proteins as a crucial enzyme for the metabolism of folate. MTHFR converts 5, 10– methylenetetrahydrofolate to 5–methyltetrahydrofolate. Then, the methionine was used by body to make proteins and other important compounds [7]. The missense mutation, C677T has become the most commonly studied one, which has been considered to influence the enzyme activity of MTHFR [8]. A string of epidemiological studies have evaluated the association between the MTHFR C677T mutation and SVT risk [9-31]. In patients with homozygous MTHFR C677T mutation, MTHFR activity is reduced, thereby resulting in an elevation of serum homocysteine concentrations of about 20% that is a well-recognized risk factor of venous and arterial thrombosis [32-34]. The role that the C677T mutation in MTHFR plays in SVT remains unclear due to this unusual thrombotic location. However, these studies have yielded conflicting results, partially because of the possible small effect of the polymorphism on SVT risk and the relatively small sample size in each of published studies.
Therefore, the aim of this study is to derive a more precise estimation of the association between the MTHFR C677T point mutation and SVT by conducting a meta-analysis of epidemiological studies on this topic and to evaluate the significance of homocysteine level in SVT.
We designed and reported the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [26].
The study search was performed independently by two authors (Li and Zhang). The most recent on-line versions of the following research databases were searched up to November 2013 without language restrictions: Wanfang, Chinese Biomedicine databases, Pub Med, Embase and Web of Knowledge. The following search terms were used to identify studies: methylenetetrahydrofolate reductase or MTHFR or MTHFR C677T, homocysteine, hyperhomocysteinemia, splanchnic vein thrombosis, portal vein thrombosis, mesenteric vein thrombosis, Budd-Chiari syndrome, gene or polymorphism or variation, mutation. All of the studies searched were retrieved, and the references cited in the studies were also reviewed to identify additional published work. Animal studies, simply commentaries, case reports and unpublished reports were excluded. Review articles were also screened to identify additional eligible studies.
We included in the systematic review full-length research studies that satisfied the following criteria: 1) prospective or casecontrol studies; 2) studies on the effect of the MTHFR polymorphism on SVT; 3) genotype frequencies available in both cases and controls; and 4) they provided sufficient published data for estimating an odds ratio(OR) with a 95% confidence interval (95%CI). Accordingly, articles were excluded using the following criteria: 1) reviews, abstracts and repeat studies; 2) studies with incomplete data or zero observations in cases or controls were excluded. For the studies with the same or overlapping data by the same authors, the most suitable study with the greatest number of subjects or the most recently published study was selected.
Two authors (Yue and Wang) independently extracted the following data from included studies: first author's name, publication date, numbers of cases and controls, country, ethnicity, study design, source of controls, and number of C677T genotypes for both cases and controls. Extracted data were compared and discrepancies resolved by discussion. When necessary, another author (Lu) was consulted to resolve the dispute.
The meta-analysis mainly was used to assess the strength of the association between the MTHFR C677T mutation and SVT risk based on the genotype frequencies in cases and controls. The effect of association was indicated as the odds ratio (OR) with the corresponding 95% confidence interval (CI). All statistical tests for this meta-analysis were performed by using Stata 12.0 (Stata Corp, College Station, USA). We pooled data using the DerSimonian-Laird random effects method, which recognizes and anchors studies as a sample of all potential studies, and incorporates an additional between-study component to the estimate of variability [35]. Therefore, the random-effects model was applied in our meta-analysis to generate a more conservative estimate of the proportion. Heterogeneity was quantified using the I2 metric, which is independent of the number of studies in the metaanalysis (I2<25% no heterogeneity; I2=25-50% moderate heterogeneity; I2>50% large or extreme heterogeneity) [36]. The bias (such as publication and location bias) was investigated by funnel plot. An asymmetric plot suggested possible publication bias. It was assessed quantitatively by a linear regression test that Egger et al defined [28]. A regression line which passes through the origin of the plot (within error limits) indicates symmetry and hence the absence of bias. Begg's test and Egger's test were used to statistically assess publication bias and a p value of <0.05 was considered significant. Statistical significance was taken as p<0.05 and all tests were two-sided.
Characteristics of Including Studies
Figure 1 shows the literature search and study selection procedures. Our literature search identified 72 publications, of which 41 were excluded after review of the title and abstract. Seven publications were further excluded after review of full-text because of the following reasons: no report of the association between MTHFRC677T polymorphism and SVT (3 studies), without case-control or TDT design (1 study), data duplication (2 studies) and lack of sufficient data (1 study). Thus, 26 publications involving 1344 cases and 2856 controls in 22 case-control studies and 2 prospective studies were ultimately included [9-26,33,34,37-40]. Of these 24 studies, 9 were conducted in Asia, 11 in Europe, 2 in America, and 2 in Africa. Studies ranged in size from 12 to 262 patients. Table 1 shows the characteristics of the included studies.
The homozygous methylene tetrahydrofolate reductase (MTHFR)/C677T polymorphism (Figure 2) was investigated in a total of 22 studies in 3773 subjects (1265 cases and 2508 controls), providing a pooled OR of 2.09 (95% CI, 1.66 to 2.64; P<0.01). There was little heterogeneity among 22 studies with this outcome (P=0.203, I2=19.6%). Visual inspection of Begg's funnel plots did not show any substantial asymmetry (z=0.31, P=0.756). Egger's regression test also indicated little evidence of publication bias (t=-0.42, P= 0.677) (Figure 3).
Sixteen studies evaluated the risk between MTHFRC677T and PVT. In subgroup analysis, MTHFRC677T was significantly higher in PVT versus controls (OR=1.73, 95% CI=1.21 to 2.48, P<0.01). There was moderate heterogeneity among 16 studies with this outcome (P=0.023, I2=45.9%). (Figure 4) Visual inspection of Begg's funnel plots did not show any substantial asymmetry (z=0.04, P=0.967). Egger's regression test also indicated little evidence of publication bias (t=1.99, P=0.065).
Only six studies evaluated the risk between MTHFRC677T and BCS. Data were obtained from 6 studies involving 318 cases with BCS and 508controls. A meta-analysis of the OR found in our current study for MTHFR/C677T in BCS generated a pooled observed OR of 1.36 (95% CI=0.61 to 3.02, P>0.05). The CIs for the expected OR fall entirely within the CI for the observed OR, suggesting a causal relation between MTHFRC677T and BCS. There was large heterogeneity among six studies with this outcome (P=0.003, I2=71.9%) (Figure 4).
The homozygous MTHFR C677T polymorphism was investigated in five studies. These totalled 998 subjects (177 mesenteric vein thrombosis cases; 821 controls) providing a pooled OR of 3.14 (95% CI=1.97 to 5.01, P<0.01) with a recessive random-effects model. There was no evidence of inter-study heterogeneity (P=0.343, I2=11.0%) (Figure 4).
Data were obtained from 6 retrospective studies (419 cases and 579 controls). The heterogeneity among studies was significant (P<0.01, I2 =57.4%). Using a random-effects model, the plasma homocysteine level was significantly higher in SVT patients than in healthy controls (SMD=0.50, 95% CI=0.12–0.87, P=0.010) (Figure 5).
The overall meta-analysis results of prospective and retrospective epidemiological studies indicated that the MTHFR C677T mutation seems to be a risk factor for SVT with little heterogeneity. This study also showed highly significant associations between homocysteine level in SVT risk. Likewise, in subgroup analysis, positive results were detected between MTHFRC677T mutation and PVT with lower heterogeneity. Similarly positive results were also found in MVT with little heterogeneity. However, the subgroup meta-analysis only comprising 4 studies showed non significant association between MTHFRC677T mutation and BCS with large heterogeneity. To the best of our knowledge, this current meta-analysis firstly integrated the prospective and casecontrol studies to reflect the precision effect of MTHFRC677T polymorphism and homocysteine level in SVT risk.
It is well known that hyperhomocysteinemia to cause venous and arterial thromboembolism. Two early meta-analysis of the results demonstrated a significantly increased risk of venous thromboembolism in patients with elevated plasma homocysteine levels [41,42]. Several investigations have shown that the thermolabile mutant of MTHFR causes moderate hyperhomocysteinemia [43]. Because the C677T point mutation in the encoding region of the MTHFR gene closely related to hyperhomocysteinemia, converting the codon for alanine to valine [44]. Meta-analysis of studies also suggested that hyperhomocysteinemia was a risk factor for venous thrombosis [45]. Our overall meta-analysis result was similar with these results.
However, caution is required when interpreting these results given the small sample size, which is inherent to the rarity of SVT. As a consequence, data obtained from this meta-analysis have to be interpreted with caution, especially for BCS and MVT. Recent studies have shown that primary BCS should be regarded as a multifactorial disease, and that co-occurrence of several prothrombotic disorders leads to thrombosis at this uncommon location. BCS is less common in western countries but primary membranous obstruction of the inferior vena cave (IVC) is the most common cause of BCS in South Africa and Asia [29,47]. MTHFR gene mutation was the first most common etiology of BCS in the current study, evident in 71.1% of patients, of whom 61.5% were heterozygotes and 9.6% homozygotes, but there was no control group [48]. In our meta-analysis, BCS was only from Asia and Europe which may affect the reliability of the results [11,13,19,26]. And as far as stratified analysis of sample size was concerned, positive results were detected in the large-sample-size subgroup but not in the small-sample-size, implying the null association was probably caused by limited sample size. These findings, in light of our meta-analysis results, highlight the need for rigorous, large-scale prospective studies that confirmed whether MTHFRC677T mutation and homocysteine play a role in the pathogenesis of the BCS.
Despite we have made great efforts to collect all possible data for investigating between MTHFRC677T polymorphism and SVT, but several potential limitations inherent in our meta-analysis should be acknowledged. First, both prospective and retrospective studies are influenced by confounding due to age, sex, smoking and body mass index. Second, most studies included in this meta-analysis involved less than a few hundred cases, and so the confidence intervals around the odds ratios were wide. Third, publication bias is a potential problem for interpretation the results in meta-analysis. When we collected full-text articles in our research, excluded abstract and unpublished articles, and positive results may be easily accepted by journals, potential publication bias may occur. Fourth, all included studies were conducted over different time periods (2000-2011); therefore, it is possible that diagnosis and treatment modalities and referral patterns may have changed over time. Fifth, a considerable heterogeneity amongst the included studies was noticed. Therefore, the random-effects model was applied in our meta-analysis to generate a more conservative estimate of the proportion.
Despite of some limitations, our meta-analysis demonstrated that MTHFR C677T polymorphism and homocysteine may be the risk factors for PVT and MVT. Subgroup meta-analysis results failed to provide compelling evidence of an association between the MTHFR C677T gene mutation and risk of BCS. Therefore, more studies with large sample sizes are required to confirm the current findings and explore the interaction between genetic polymorphism, homocysteine and SVT risk.
The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the manuscript and its final contents. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript. The authors have no conflicts of interest
 |
| Figure 1: Flow chart of the retrieved steps of our meta-analysis |
 |
| Figure 2: Forest plot assessing the potential association between MTHFRC677T and risk of SVT in all included studies |
 |
| Figure 3: Funnel plot of MTHFR C677T point mutation and risk of SVT |
 |
| Figure 4: Forest plot assessing the potential association between MTHFRC677T and risk of PVT, BCS and MVT in all included studies |
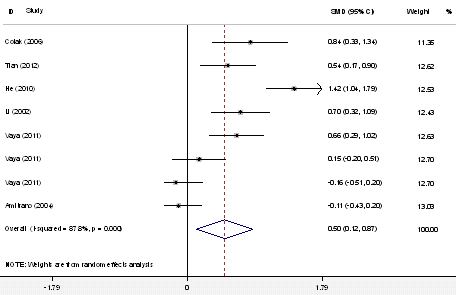 |
| Figure 5: Forest plot assessing the potential association between homocysteine and risk of SVT in all included studies |
| Author,Year | Ethnicity | Study | N(Ca/Con) | Case/ Control | Sex (M,F) |
|---|---|---|---|---|---|
| Amitrano et al,2000 | Europe/ Italy | case-control | 23/471 | PVT/ healthy subjects | NA |
| Amitrano et al,2001 | Europe/ Italy | case-control | 12/431 | MVT/ healthy subjects | (8,4)/(244,187) |
| Amitrano et al,2003 | Europe/ Italy | case-control | 17/244 | SVT/ healthy subjects and non-thrombotic | (5,12)/(NA) |
| Heller et al,2000 | Europe/Germany | case-control | 24/100 | PVT/ healthy neonates and infants | NA |
| Li et al,2002 | Asia/ China | case-control | 41/80 | BCS/ healthy neonates and infants | (30,11)/( 51,29) |
| Pinto et al,2003 | Americas/Brazil | case-control | 14/28 | PVT/ healthy, age- and sex-matched individuals | NA |
| Pinto et al,2004 | Americas/Brazil | prospective | 14/28 | PVT/children without hepatopath | (7,7)/(NA) |
| Saxena et al,2004 | Asia /India | case-control | 105/68 | BCS and PVT/ control group free of liver disease | (64,51)/( NA) |
| Erkan et al,2005 | Asia/ Turkey | case-control | 36/80 | PVT/ healthy age- and sex-matched persons | (22,14)/(46,34) |
| Mangia et al,2005 | Europe/ Italy | prospective | 43/176 | PVT/ healthy volunteers | (22,21)/( 90,86) |
| Agaoglu et al,2005 | Asia/ Turkey | case-control | 28/103 | MVT/ cirrhotic patients without PVT | (16,12)/(60,43) |
| Pasta et al,2006 | Europe/ Italy | case-control | 78/78 | PVT/ healthy controls | (50,28)/( 55,23) |
| D'Amico et al,2009 | Europe/ Italy | case-control | 94/94 | PVT/ cirrhotic patients without PVT | NA |
| Gabr et al,2010 | Africa /Egypt | case-control | 76/20 | PVT/ healthy age- and sex- matched volunteers | (47,29)/(10,10) |
| He et al,2010 | Asia/ China | case-control | 63/128 | MVT/ sex- and age-matched healthy individuals | (49,14)/(95,33) |
| Vaya et al,2011 | Europe/Spain | case-control | 48/84 | PVT,BCS and MVT/ healthy individuals | (26,22)/(NA) |
| Hoekstra et al,2010 | Europe/7countries | case-control | 262/116 | PVT and BCS/ Healthy controls | (119,143)/(NA) |
| Amitrano et al,2004 | Europe/ Italy | case-control | 79/79 | LC with PVT/ LC without PVT | (47,32)/( 47,32) |
| Chamouard et al,2001 | Europe /France | case-control | 22/60 | PVT without LC/ DVT | NA |
| Colak et al.,2006 | Asia/ Turkey | case-control | 32/33 | BCS/ Healthy controls | (16,16)/( 15,18) |
| Hu et al,2008 | Asia/ China | case-control | 63/75 | MVT/ healthy individuals | (49,14)/(56,19) |
| Maras et al,2010 | Asia /India | case-control | 70/200 | LC with PVT/ LC without PVT | NA |
| Salama et al,2007 | Africa /Egypti | case-control | 40/20 | PVT without LC/ Healthy controls | (27,13)/( 14,6) |
| Tian et al,2012 | Asia/ China | case-control | 60/60 | BCS/Healthy controls | ( 33,27)/(28,32) |
| NA, not available; Table 1: Baseline characteristics of studies included in the meta-analysis |
|||||






































