Top Links
Journal of Case Reports and Studies
ISSN: 2348-9820
An Observational Study on the Clinical Efficacy of Neramexane in Subjective Tinnitus in a Phase 2 Clinical Trial in a Hispanic Cohort in Laredo, Texas
Copyright: © 2017 Soriano R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
An observational study was conducted on the efficacy of Neramexane in a small cohort of Hispanic subjects who were diagnosed with moderate to severe subjective tinnitus. Patients were screened using the inclusion and exclusion criteria as outlined by the study sponsor. The study protocol involved daily intake of Neramexane 50 mg/day (for weigh<90 kg) or 75 mg/day (weight>90 kg) for 29 weeks with primary efficacy assessed through audiogram study and 3 electronic self-reporting questionnaires: Tinnitus Handicap Inventory-12 (TBF/THI-12) Scale, Tinnitus Rating Scale (TRS) and Hospital Anxiety and Depression Scale (HADS). Audiogram studies were conducted at baseline and at subsequent visits to document hearing level and progress with treatment. Comparative analysis with the subjective outcome measures (3 self-reporting questionnaires) in the cohort group of 14 patients indicate 75% improved in their subjective tinnitus after 29 weeks of Neramexane intake. The same patients that reported improved subjective tinnitus also demonstrated improved hearing. There was correlation of improved audiogram results with improved scores on the THI-12, TRS and HADS. These findings suggest potential efficacy of Neramexane in the improvement of hearing in those with moderate to severe subjective tinnitus. Associated comorbid conditions for tinnitus include hypertension, hyperthyroidism and vertigo. Predisposing occupational risk factors and exposure to chronic ear trauma support current knowledge about tinnitus.
Keywords: Tinnitus; Neramexane; CohortTinnitus, as defined by National Institute on Deafness and Other Communication Disorders (NIDCD), is a symptom of hearing loss characterized by “ringing of the ears” but also roaring, hissing or buzzing sounds in one or both ears.1 It has been reported to affect 10% of Americans, nearly 24 million [1]. As such, the increased prevalence impacts the quality of life especially of the aging population.
There are several causes of tinnitus of which noise-induced hearing loss in the workplace or occupation produces progressive sensory damage in the inner ear [1]. Associated medical conditions and medications have been reported to cause hearing loss and tinnitus [1].
It has been reported with sudden onset of high blood pressure and chronic intake of aspirin and quinine. There are different postulated mechanisms of this disorder with damage to the peripheral and central auditory pathways [2]. A novel theory was proposed by Leaver et al. in 2011 [2]. Their study identified functional and anatomical anomalies in the primary and posterior auditory cortices and anomalies of 2 the limbic structures nucleus accumbens and ventromedial prefrontal cortex. The limbic system is a network of brain structures involved in emotion, behavior and long term memory [2]. The researchers detected hyperactivity of the nucleus accumbens and structural differences in the ventromedial prefrontal cortex. It was proposed that a dysregulation of interaction between the limbic and auditory cortex occurs in tinnitus suggesting the breakdown of the limbic system as the gatekeeper of conscious perception of sounds [2]. In severe tinnitus, patients have associated depression or sleeping difficulties. This may produce a vicious circle leading to a progressive deterioration of quality of life and more abnormal processing of signal in the sensory system [3,4].
There are no standard approved pharmacological treatments for tinnitus. Neramexane (1-amino-1,3,3,5,5-pentamethylcyclohexane mesylate) is a novel compound antagonist to both α 9/α 10 nicotinic (acetylcholine) receptors and N-methyl-D-aspartate (NMDAR) receptors [2,5-7]. It also has antagonistic properties to 5-hydroxytryptamin (5-HT3, serotonin) receptors.
Pharmacological studies have demonstrated that Neramexane may be useful for the treatment of a wide range of central nervous system (CNS) disorders involving a dysfunction of cholinergic and glutamatergic neurotransmission (Eggermont). NMDAR provide the basis for the neurophysiologic model of tinnitus where auditory, limbic and autonomic nervous systems interconnect. This similar mechanism explains how NMDAR antagonists protects from hearing loss from free-radical-induced damage to hair cells from a variety of causes such as use of aminoglycosides, acoustic trauma or cochlear ischemia [5,8]. Neramexame has the ability to regulate the imbalance of inhibitory and excitatory neurotransmission in the autonomic and limbic systems which are postulated to cause tinnitus [2].
The safety, tolerability and pharmacokinetic profile of Neramexane have been investigated in a number of studies but by and large show tolerability without safety concerns [8]. The incidence of adverse events such as dizziness and vertigo usually occur initially but decrease thereafter. Neramexane did not show an adverse influence on vital organ function at pharmacologically relevant dose levels [8].
Manufacturer data indicate that the 50 mg per day dose showed the best efficacy and slightly better tolerability than the 75 mg per day dose. However patients weighing more than 90 kg benefited more from the higher (75 mg) dose while showing considerably less side effects: 12.5% dizziness in the > 90 kg group versus 19.6% dizziness in the group receiving 50 mg neramexane per day. Henceforth, the target daily dose was 75 mg Neramexane for patients weighing ≥ 90 kg and 50 mg Neramexane for patients weighing < 90 kg.
It is important to differentiate between objective and subjective tinnitus. In more than 95% of cases, the perceived tinnitus is purely subjective in nature without physical source of the acoustic signal. In the remainder of cases, as in objective tinnitus, the patient’s perception of sound is caused by a real source of sound waves. Subjective tinnitus has been defined as the perception of a sound which results exclusively from the activity within the nervous system without any corresponding mechanical, vibratory activity within the cochlea [9]. It is defined as an auditory “phantom perception” without internal or external stimuli [9,10]. It may consequently be tolerable or debilitating.
The diagnosis of tinnitus is mainly a clinical one and audiograms coupled with patient interviews and multidimensional tinnitus questionnaires and rating scales are used clinically to characterize its severity and impact on daily living [11-14]. There are no well-established, specific medical treatments for tinnitus. A combination of treatments such as hearing aids, cochlear implants, wearable and tabletop sound generators, acoustic neural stimulation and medications such as antidepressants and anxiolytics have been used with varying efficacy [1].
This single-arm study was part of a phase 2, open-label, pharmacokinetic and pharmacodynamic multicenter studies in subjects with subjective tinnitus. Subjects and investigators had knowledge of Neramexane treatment.
Patients aged 18-75 years with the diagnosis of first-onset uni- or bilateral persistent moderate to severe tinnitus of 3-12 months duration and fulfilling the inclusion criteria outlined in Table 2 were recruited to the study. The research protocol in Table 1 and Figure 1 details the study. Main inclusion criteria consisted of the following scores: THI-12 > 9, TRS > 4, and HADS <10 for both anxiety and depression. Audiogram testing at baseline and subsequent visits were done to document level of hearing or hearing loss. THI-12, TRS and HADS questionnaires were administered at screening or baseline periods, 17 weeks and 29 weeks of treatment via patient electronic diaries returned directly to the study sponsor for downloading.
Neramexane at a dose of 50 mg (<90 kg patients) or 75 mg (patients >90Kg) was self administered. There were a total of 9 patient visits from screening to 30-35 days after end of treatment (EOT). At each visit safety checks were conducted consisting of: monitoring for adverse events, laboratory testing (biochemistry, hematology, coagulation and urine analysis), 12-lead electrocardiogram, (ECG), vital signs, physical examination, ophthalmological examination, questioning for concomitant medications, treatments and sexual dysfunction. The duration of treatment for both titration and fixed dose periods was 29 weeks. Therapeutic blood levels of the Neramexane were consistently monitored during the titration and fixed dose periods of the study (Visits 2-8; Figure 2 Study Design).
Neramexane mesylate, 12.5 mg, 25 mg, and 37.5 mg immediate release (IR) film-coated tablets for oral administration was administered at a daily dose 50 mg (given as 25 mg twice daily), or 75 mg/day (given as 37.5 mg twice daily) for patients weighing ≥ 90 kg. Neramexane was slowly up-titrated over 4-5 weeks (depending on the target daily dose) to improve tolerability. Patients experiencing subjective or objective intolerance symptoms with the target dose of 75 mg per day were allowed to reduce the dose to 50 mg per day. However, rechallenge back to 75 mg per day again was not allowed. Patients not tolerating a daily dose of 50 mg were discontinued from the study.
Exclusion Criteria: Patients with clinical diagnosis of intermittent or pulsatile tinnitus or with an underlying otological or neurological disease or conductive hearing loss in at least 1 ear; patients with hearing aids less than 6 months before screening; patients with active uncontrolled systemic disease of other organ systems or malignancy; prior use of Neramexane or use of medications that are intended to treat tinnitus; history of hypersensitivity to Neramaxane or any of of its ingredients; intake of unauthorized medications; patients with history of alcohol or drug abuse in the last 3 years; systolic BP >180 mmHg or <90 mmHg or diastolic BP>105 mmHg or <45 mmHg; and those scheduled to undergo elective surgery.
Outcome Measures: Absolute changes in 3 self-reporting scales were used to evaluate improvement in subjective tinnitus: THI-12, TRS and HADS. The THI-12 (Tinnitus Handicap Inventory-12) is adapted from the German version called TBF-12 and the original English THI (Tinnitus Handicap Inventory) (Table 3). It is a self-report questionnaire assessing 12 items from 2 dimensions: emotional-cognitive factors and functional-communicational factors. Each item is rated with 0-2 (2= “often”; 1= “sometimes”; 0= “never”). The maximum score is 24 indicating most severe tinnitus impairment. The Tinnitus Rating Scale (TRS) is an 11-point Likert-like scale asking for the past week (Table 4). The scores range from 0 (indicating no tinnitus) to the maximum score of 10 (characterizing the most severe tinnitus considered). The Hospital Anxiety and Depression scale is a 14-questionnaire test of which 7 are directly assessing anxiety and another 7 for depression (Table 5). At any point scores above 11 for anxiety or depression were considered abnormal and indicative of a possible psychological dysfunction that warranted exclusion.
The HADS [15] is a screening tool for anxiety and depression in non-psychiatric clinical population. The scale consists of 14 items (7 each for anxiety and depression). Each item is rated on a four point scale ranging from 0 (not at all) to 3 (very often). Responses are based on the relative frequency of symptoms over the preceding week.
Globally, there were 431 outpatient subjects with moderate to severe tinnitus recruited for this phase 2 clinical trial. At our site, 14 Hispanic adult patients, ages 18-75 years old, met the inclusion criteria and none of the exclusion criteria. Of the 14 patients, 12 were females, and 2 were males. Comparison of 4 outcome measures from screening or baseline studies to 17 weeks and 29 weeks end of treatment documented consistent improvement in 75% (10 out of 14) of patients as reported in their self-reporting questionnaires THI-12, TRS, and HADS which were downloaded electronically off-site by the study sponsor. After 29 weeks of intake of Neramexane, comparison of audiograms measured during the screening period, week 2 and end of treatment showed consistent improvement of hearing compatible with the reported subjective improvement in the 3 self-reporting questionnaires. The same 10 patients that had improved audiograms demonstrated the same absolute change in the 3 outcome measures in their electronic self-reporting questionnaires indicating improved quality of life and psychological well-being. The anxiety and depression levels improved.
During the entire 29 weeks of study, Neramexane did not produce any adverse events nor abnormal clinical biochemistry, hematology, coagulation and urine analysis. Electrocardiograms (12-lead ECG) and ophthalmologic screenings remained normal at all designated visit days. Physical examination of the patients remained stable throughout the entire study period.
In analyzing patient characteristics, we noted the presence of comorbid conditions that did not warrant exclusion. They were hypertension (70%), hyperthyroidism (70%) and benign positional vertigo (50%). Factors that contributed heavily to the onset of tinnitus in some of these affected individuals included chronic ear trauma, traumatic perforation of the tympanic membrane and constant exposure to high-volume noise such as from occupational exposure to gun shots by police officers and military personnel and hearing loud music at concerts.
Acetylcholine and N-methyl-D-aspartate (NMDA) receptors are thought to play important roles in the development of tinnitus. Disturbances of glutamatergic transmission have been implicated in various disorders of the central nervous system as well tinnitus [2,5-7,16-19]. These alterations change the balance between excitatory and inhibitory brain processes in the auditory pathway, but are also likely to involve other brain structures, namely the limbic system. NMDA-receptor-relayed projections from the medial geniculate body to the lateral nucleus of the amygdala play important role in the interconnections between the auditory, limbic and autonomic nervous support the theory that brain structures other than the auditory pathway must be involved.
NMDA receptor antagonists have been reported to afford protection from hearing loss caused by free-radical-induced damage to the hair cells. The nicotinic acetylcholine receptor subunits play a relevant role in the efferent auditory system and the medial olivocochlear pathway. Activation of the nicotinic acetylcholine receptor subunits inhibits mechanical amplification brought about by the activity of outer hair cells. As this nicotinic/cholinergic pathway becomes imbalanced, Neramexane may provide therapeutic intervention as it targets NMDA receptors. Neramexane has moderate-affinity, non-competitive NMDA receptor antagonist activity. It is also postulated to have antagonistic properties to 5-hydroytryptamin (5-HT3, serotonin) receptor, another mechanism of action in tinnitus [16]. It has been found to have good efficacy and tolerability.
We have described a small cohort of Hispanic patients with moderate to severe tinnitus that demonstrated improved patient outcomes measured by the THF-12, TSS and HADS questionnaires. Although tinnitus is a subjective symptom, the improvement in the patient’s general well-being was evidenced by decreased anxiety and depression. The secondary result of improved hearing on audiogram studies after a 29 week intake of Neramexane at the dose recommended by the study sponsor is promising of its potential in the treatment of hearing loss.
It has been summarized that the tinnitus produces distress in 5 main categories [20]: 1) emotional disturbance and patient’s view of tinnitus 2) sleep disturbance 3) auditory perceptual difficulties 4) interference with work and leisure and 5) effects on general health. The current study proves valuable in the treatment of tinnitus as it greatly affects the aging population and their quality of life. Meikle et al however detailed 19 negative effects of tinnitus10: 1) sleep disturbance 2) difficulty concentrating 3) difficulty ignoring tinnitus 4) irritability and nervousness 5) tension and stress 6) reduced quality of life 7) intereference with relaxing 8) interference with quiet leisure activities 9) interference with social activities 10) depression 11) anxiety 12) interference with work 13) anger, frustration, annoyance 14) discomfort in quiet 15) reduced sense of control 16) inability to cope 17) interference with hearing 18) feeling tired, ill or fatigued 19) unhappiness and distress.
In this described adult cohort of Hispanics, we noted a high percentage of patients in with hypertension and tinnitus. This may be explained by the activation of the sympathetic nervous system (SNS), in particular, the fight or flight response [21]. This in turn induces a high frequency of metabolic and brain activity, which may lead the brain to generate signals originating from the cerebral cortex resulting in detrimental auditory sounds that are completely unrelated to the outside environment. This could also be specifically attributed to the high frequency of action potentials transmitted to the brain for neurological processing. Roberts et al. [22] noted thru neuroimaging studies, an in increased firing rate and hyperactivity of the central auditory system in tinnitus. The spontaneous increased firing rate is thought to be from reduced inhibition from decreased output of the cochlear region.
Impaired vasomotor regulation in hypertensive and hyperthyroid conditions imply disturbance of cochlear microcirculation that results in intermittent labyrinthine functional damage, henceforth visuospatial disturbances such as vertigo that contribute to the severity of tinnitus [23]. The presence of vertigo and headaches may partially explain the subjective response of tinnitus as unequal, constant or intermittent. It has been described that approximately 19% of patients with positional vertigo reported concurrent tinnitus suggesting a vestibular origin of tinnitus.
In hyperthyroidism, the excess secretion of thyroid hormones may lead to more sensory activation of the emotional and mental faculties of humans implying that increased mood manifestation causes the brain to generate a higher frequency of neurotransmitter firing and as result induce the ringing and buzzing sound characteristic of tinnitus [24]. This mood change is consistent with the improved anxiety and depression scores in those whose tinnitus improved with Neramexane, thereby suggesting interruption of the vicious circle of abnormal alteration of sensory signals [3,4]. This observation supports the role of the limbic system in modulating or perpetuating tinnitus [9].
The 29 week observational study supports prior results described in the literature that demonstrates the safety and efficacy of Neramexane in the treatment of moderate to severe tinnitus [16]. The majority of patients demonstrated improved hearing via audiogram studies. The improved hearing correlated with the 3 self-reporting scales that assessed the patients’ general well-being and quality of life. This study demonstrated the correlation of the objective measure with the patients’ subjective self-assessment of improved attention, coping or frustration level, concentration, job and household responsibilities, social activities and relationships with family and friends. The impact of Neramexane’s efficacy on the patients’ quality of life was manifested by overall decreased anxiety and depression levels.
We believe that the Tinnitus Handicap Inventory-12, Tinnitus Rating Scale and Hospital Anxiety and Depression Scale are reliable parameters that can be used for future studies assessing the impact of Neramexane on patients’ ability to cope with the demands of daily in those impaired with neurological or otological disease.
Although there is no medication known to improve hearing loss, we have demonstrated the potential use of Neramexane in improving hearing in patients with moderate to severe tinnitus. Larger double-blind placebo controlled studies are needed to determine its efficacy in treating those afflicted with tinnitus and hearing loss.
The effective management of tinnitus requires a multi-dimensional approach. The incidence and relationship of tinnitus in comorbid conditions such as benign essential hypertension, hyperthyroidism and benign positional vertigo are worth further investigation. More behavioral studies targeting amelioration of symptoms will help improve the quality of life as the patients cope with the debilitating condition. Hopefully, the affected patient’s reports of subjective tinnitus in the questionnaires for outcome measures will similarly improve. We postulate the possible role of cultural perceptions that may affect reporting of subjective tinnitus. We suggest characterization of cultural differences as they relate to the self-evaluation of the rating scales of the outcome measures. Ethnicity and socio-cultural perceptions are important variables in self-assessment studies. Our study was conducted in Laredo, Texas, a predominantly Hispanic community. Henceforth, implications of our findings cannot be generalized.
Additional questioning of the duration and severity of occupational exposure to noxious environmental stimuli are important risk factors that warrant further studies so that occupational hazards and workplace policies can be modified accordingly.
This study was conducted in accordance with applicable laws and regulations including, but not limited to, the International Conference on Harmonisation (ICH), Guideline for Good Clinical Practice (GCP), and ethical principles that have their origins in the Declaration of Helsinki. The institutional review board (IRB) reviewed and approved the protocol and informed consent form (ICF) before any subjects were enrolled. Before any protocol-required procedures were performed, the subjects signed and dated IRB-approved ICF.
Investigational medicinal drug Neramexane supplied by Merz Pharmaceuticals, Frankfurt, Germany. Research facility provided by Envision Clinical Research, LLC, 401 Westmont Laredo, Texas 78041.
(Permission requested Jih-Heng Li, Substance Abuse and Rehabilitation 2011:2 11-20) |
Figure 2: Study Design |
Patient population, diagnosis and main criteria for inclusion |
Patient population and diagnosis: Outpatients aged = 18 and = 75 years with a diagnosis of first onset, persistent, uni- or bilateral moderate to severe subjective tinnitus of 3-12 months duration Main inclusion criteria: • Tinnitus duration = 3 and = 12 months at Screening (Visit 1) • Tinnitus Handicap Inventory-12, total score = 9 at Screening (Visit 1) and Baseline (Visit 2) • Tinnitus Rating Scale, one week version = 4 at Screening (Visit 1) and Baseline (Visit 2) • Hospital Anxiety and Depression Scale (HADS) depression and anxiety subscores = 10 at Screening (Visit 1) |
|---|---|
Number of visits |
9 visits: Screening, Baseline, Week 2, Week 5, Week 11, Week 17, Week 23, and Week 29 plus a safety follow-up visit 30-35 days after end of treatment |
Duration of treatment per patient |
29 weeks treatment with Neramexane including a 4- or 5-week up-titration period, depending on the target daily study drug dose (50 mg for body weight < 90 kg or 75 mg for body weight ≥ 90 kg) |
Evaluation criteria |
Outcome Measures: • THF-12 total score absolute change from Baseline to Week 17 and 29 or to premature discontinuation if patients ends treatment before Week 17 or 29 • Tinnitus Rating Scale absolute change from Baseline to Week 17 and 29 or to premature discontinuation if patient ends treatment before Week 17 or 29 • HADS Hospital Anxiety and Depression Scale absolute change from Baseline to Week 17 and 29 or to premature discontinuation if patients ends treatment before Week 17 or 29 • Audiogram testing during the screening period (between Visit 1 and Visit 2) after Week 2 (Visit 3) and at the end of treatment (Visit 8) Safety: • Adverse Events (AEs) - voluntary reporting by patients and inquiry by the investigator • Clinical biochemistry, hematology, coagulation and urine analysis • 12-lead ECG at Visit 1, Visit 4, Visit 6 and Visit 8 • Vital signs (pulse rate, blood pressure) • Physical examination • Ophthalmologic examination at Visit 1 and Visit 8 • Concomitant medications • Concomitant treatments • Inquiry about sexual dysfunction |
Table 1: Research Protocol |
|
Inclusion Criteria |
Screening
Visit 1
|
Baseline
Visit 2
|
|---|---|---|
1. Signed written informed consent obtained from the patient |
X |
|
2. Patients with a clinical diagnosis of first onset, persistent (i.e. tinnitus should never be absent for > 24 hours in a row), subjective, uni- or bilateral tinnitus present for at least 3 months but not more than 12 months. In case of bilateral tinnitus this criterion applies to both ears. |
X |
|
3. Outpatients between 18 and 75 years of age (inclusively) at Screening |
X |
|
4. For females of childbearing potential (last menses less than one year prior to enrolment): negative pregnancy test at Screening and at Baseline (i.e. prior to entry in the double-blind treatment phase); not breast-feeding; either surgically sterile or agreement to use a medically accepted, effective contraception during the entire duration of the study. An effective method of birth control is defined as those, alone or in combination, which result in a low failure rate when used consistently and correctly such as implants, injectables, combined oral contraceptives, non-hormonal IUDs, sexual abstinence or vasectomised partner or double contraception methods (e.g. condome with spermicide cream). |
X |
X |
5. THI-12 total score ≥ 9 and TRS ≥ 4 |
X |
X |
6. HADS depression subscore ≤ 10 and HADS anxiety subscore ≤ 10 |
X |
|
7. Physical examination and laboratory evaluations from the Screening visit must be normal, or abnormal findings must be judged either “not clinically relevant” or “clinically relevant but of no concern” by the Investigator |
X |
|
8. Patient must be willing and able to comply with the protocol and study
procedures
|
X |
X |
| Note: X = performed
Table 2: Inclusion Criteria |
||
 |
2= “often”; 1= “sometimes”; 0= “never” |
 |
Table 4: Tinnitus Rating Scale |
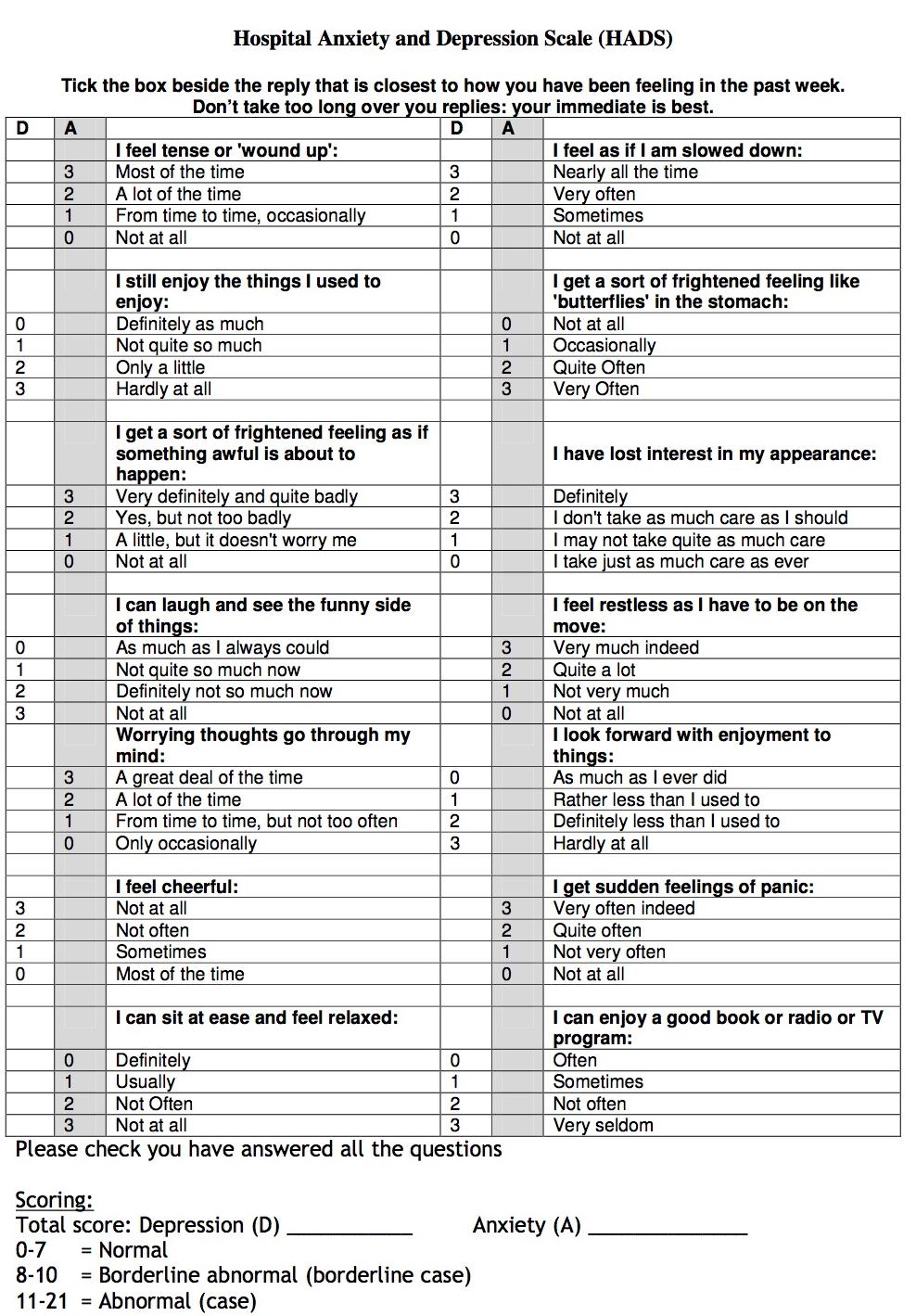 |
Table 5: Hospital Anxiety and Depression Scale (HADS) |






































