Top Links
Journal of Biostatistics and Biometric Applications
ISSN: 2455-765X
Effect of Fe2+, Mn2+ Catalyst on the Performance of Bio-Electro-Fenton Microbial Fuel Cells
Copyright: © 2015 Kao SW. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
The Bio-electro-Fenton microbial fuel cell (BeF-MFC) system is the new energy efficient environmental technology being extensively studied. This is because organic matter being degraded in an anode cathode chamber will simultaneously produce electricity and reduce the processing expenses of waste treatment. Concerning the effect of catalysts applied in Bio-electro-Fenton systems on strengthening the performance, in this study two kinds of catalysts, Fe2+ and Mn2+, will be used in the Bio-electro-Fenton system. The aim is to ascertain their performance on the degradation of dairy wastewater in the anode, and oily wastewater in the cathode part, respectively. Results show that 40%/4hr of COD degradation can be arrived at in the condition of the Fe2+ catalyst but is ineffective for the Mn2+ catalyst. The findings in this study will provide useful information for the improvement of Bio-electro-Fenton microbial fuel cells in the future.
Keywords: Microbial fuel cell; Bio-electro-Fenton; Wastewater; Degradation; Catalyst
In the study of Bio-electro-Fenton microbial fuel cells [1-6], many studies have demonstrated that Fenton reactions will have a significant influence in the degradation of organic pollutants [7-12], and also have higher reaction efficiencies [13-16]. Zhu Xiuping et al. initiated Bio-electro-Fenton MFC research. In their study carbon felt was used for the anode and cathode electrodes and sludge from sewage treatment plants was embedded in the anode chamber, with glucose being used as organic matter (1-1) [7]. Li Zhuang showed that an electro-Fenton (EF) reaction impels a cathodic reaction in MFCs because the cathode chamber undergoing continued aeration by air, and H+, O2, e- produces H2O2 (1-2), in acidic conditions [10]. H2O2 reacts with Fe2+ to produce hydroxyl radical (OH•) (1-3)(1-4), which has high activity and a high oxide level [8,9], to increase the competitiveness of the electrochemical wastewater treatment [16]. M. Panizza and G. Cerisola did an experiment that exhibited that the presence of Fe2+ greatly improved COD removal up to more than 90%, so the catalyst would impact the COD removal itself [16].
In Table 1, electro-Fenton systems are single electrolites and use precious metals. However, in this study MFCs did not use precious metals [17,18] and two chamber MFCs were used as a method to degrade pollutants using electro-Fenton reactions (Table 1). However, few studies related to MFCs by the Bio-electro-Fenton system process with Fe2+, Mn2+ as the catalyst are to be found [8,18-20]. B. Balci noted that using Fe2+ catalyst can cause iron-chelating to occur and also induce an unstable reaction with organic matter [18]. Therefore, usage of a Mn2+-mediated EF process, shown in equations (1-5) to (1-7), would be suggested and utilized in the study.

As the features of the Mn3+ have a higher standard reduction potential accepting electrons than Fe3+, the regeneration of Mn2+ reacting with hydrogen peroxide for producing hydroxyl radicals will be faster [17]. In this study the improved catalyst will contribute to degradable efficiency in the electro-Fenton system reaction. Concerning the effect of catalysts applied in BeF systems on strengthening the performance, in this study two kinds of catalysts, Fe2+ and Mn2+, were used in the Bio-electro-Fenton system to ascertain their performance on the degradation of dairy wastewater. This can generate electricity [21] in the anode and oily wastewater, which will pollute the water resource in the cathode part, respectively.
The BeF-MFCs used a dual-chamber acrylic square because it is easy to observe (Figure 1) with a cell system of 1.5 liters (130 mm×110 cm×130 mm). The carbon felt (8 cm long, 4 cm wide) was used for the electrodes which had been treated [9]. A Nafion-117 membrane, whose total reaction area is 7.2×103 mm2 (DuPont Limited USA), was used between the anode and the cathode to increase H+ diffusion. Dairy wastewater with a volume of 1 L was taken from one of the dairy companies and set at an anaerobic fermentation condition ranging from six months to three years and was utilized in this study. It was used as the anode liquid, and the oily wastewater (1 mL diesel oil + 1 L water + 10 g emulsion) was added to the cathode. The two kinds of catalyst 0.75 mM FeSO4•7H2O and MnSO4•H2O, which are granules of solid solutions, acceded to the oily wastewater in the cathode and an aquarium pump allowed continued aeration [22]. The cell voltages and polarization curve of the BeF-MFCs were recorded for 5 days by using a data acquisition system (model 5020 Jiehan instrument) at the external resisters of 1000 Ω and the expression of performance of electrochemical analyzers (Jiehan ECW-5600 Taiwan) measured experiment. The cathode solution was diluted 100 times. Similarly, the study was executed on matching the multifunctional water analysis (V2000 photometer SUNTEX) with the cathode solution diluted 100 times. COD and H2O2 in the cathode chamber were analyzed for the reaction performance.
In this study, dual-chamber BeF-MFCs were incubated for one week at an external resistance of 1.0 ×103 Ω for the case of Fe2+/ Mn2+, respectively. The anode generated electricity and the cathode reacted to the BeF process. Figure 2 shows that the initial voltage and maximum voltage of BeF-MFC with Fe2+ is 0.54 V. The voltage with unsteadiness would decrease fast because of the Iron-chelating effect [23] (Figure 2). Therefore, the Fe2+ catalyst in the BeF-MFC is no longer available and effective. Conversely, BeF-MFC with Mn2+ would produce a worse power performance because a large internal resistance existed in the system.
On the power performance of BeF-MFC, Figure 3 shows that the system with Fe2+ is better than in the case of with Mn2+ because the open voltage and maximum power density is 0.3 V and 102 mW/m2, respectively (Figure 3). Conversely, Figure 4 shows that the open voltage of the system with Mn2+ is 0.24 V and 1.5 mW/m2 for maximum power density (Figure 4). The effect of Mn2+ in the system was destroyed by the factor of ohmic polarization because it could not provide enough elections for the reaction in the cathode [24], which then resulted in a poor power performance. In the low current region shown in Figure 3, an unsteady variation of the I-V curve could be found because of insufficient activity [25]. That being said, these findings of a catalyst with high activity samples of Mn5+/Mn6+ taken should be necessary for replacing the present material of Mn2+ [26].
It is well-known that COD is also an important factor in BeF-MFCs to realize their performance. In this study the relation between the concentration of H2O2 and COD was investigated [16] and is shown in Figure 5. In ideal conditions, when H2O2 and Fe2+/Mn2+ interacted in the cathode chamber, there was an abundance of OH• exchanged by the Bio-electro-Fenton (1-3), (1-6) and Fe3+/Mn3+ received the electrons to reduce the Fe2+/Mn2+ (Figure 5). The productivity of OH• will further affect the variation of COD within the BeF-MFC system. The results in Figure 5 show that the COD of the BeF-MFCs with Mn2+ would not decrease because the amount of electrons generated were too insufficient to produce H2O2, confirmed by Figure 2. Conversely, Table 1 shows that the degradation performance of COD is 40%/4hr for the case of with Fe2+ whose performance is better than the case of with Mn2+.
Table 1 was made to show the comparison between this study and other kinds of electro-Fenton for understanding the difference in numbers of the system chamber, bio-modules and usage of precious metals (Table 1). From Table 1, most of the studies shown would utilize a single-chamber electro-Fenton and afford constant-current electrolysis to provide enough electrons but did not do so in this study [5,17,18]. The author considers that the anode (dairy wastewater), cathode (oily wastewater), electrode materials [1,27] and catalyst had a direct influence in this system. When the cathode continued the BeF reaction using Fe2+, electrons would be produced in part of the anode. Results show a great effect in appearance of the voltage of cells, electrical performance, H2O2, and COD. On the contrary, the electrons could not be produced in part of the anode when the cathode would not be continuing the BeF reaction the case of using Mn2+. In this study a dual-chamber BeF-MFC, whose working principal is different to reports cited in Table 1. The carbon felt without precious metals was used in the anode. In BeF-MFC microorganisms would be used to support the generation of electricity with a function different to electro-Fenton. The BeF-MFC is independent and self-sufficient. Contrarily, many electro-Fentons needed extra power. However, electrical instability and high resistance still occurred in the case of with Fe2+/Mn2+, and findings of a catalyst with high activity should be necessary as a replacement for the present Fe2+/Mn2+ material of in the future [26].
In this study two kinds of catalyst, Fe2+ and Mn2+, were utilized in BeF-MFCs for realizing their performance on the degradation of dairy wastewater in anode and oily wastewater in cathode, respectively. Results could be summarized and are addressed as follows:
Firstly, the performance of a BeF-MFC with Fe2+ is better than in the case of with Mn2+ because the OCV and maximum power density is 18 times and 68 times that of the case of with Mn2+, respectively. Secondly, the degradation performance of COD is 40%/4hr for the case of with Fe2+. Nevertheless, the dual-chamber BeF-MFC is a complex system and also needs more effort on improving the ability of the electrode plate and catalyst for enhancing the performance of the BeF-MFC system in the future.
Authors acknowledge support from the Ministry of Science and Technology (MOST) by Award (103-2623-E-197 -001 –ET and 103-2622-E-197 -003 -CC3).
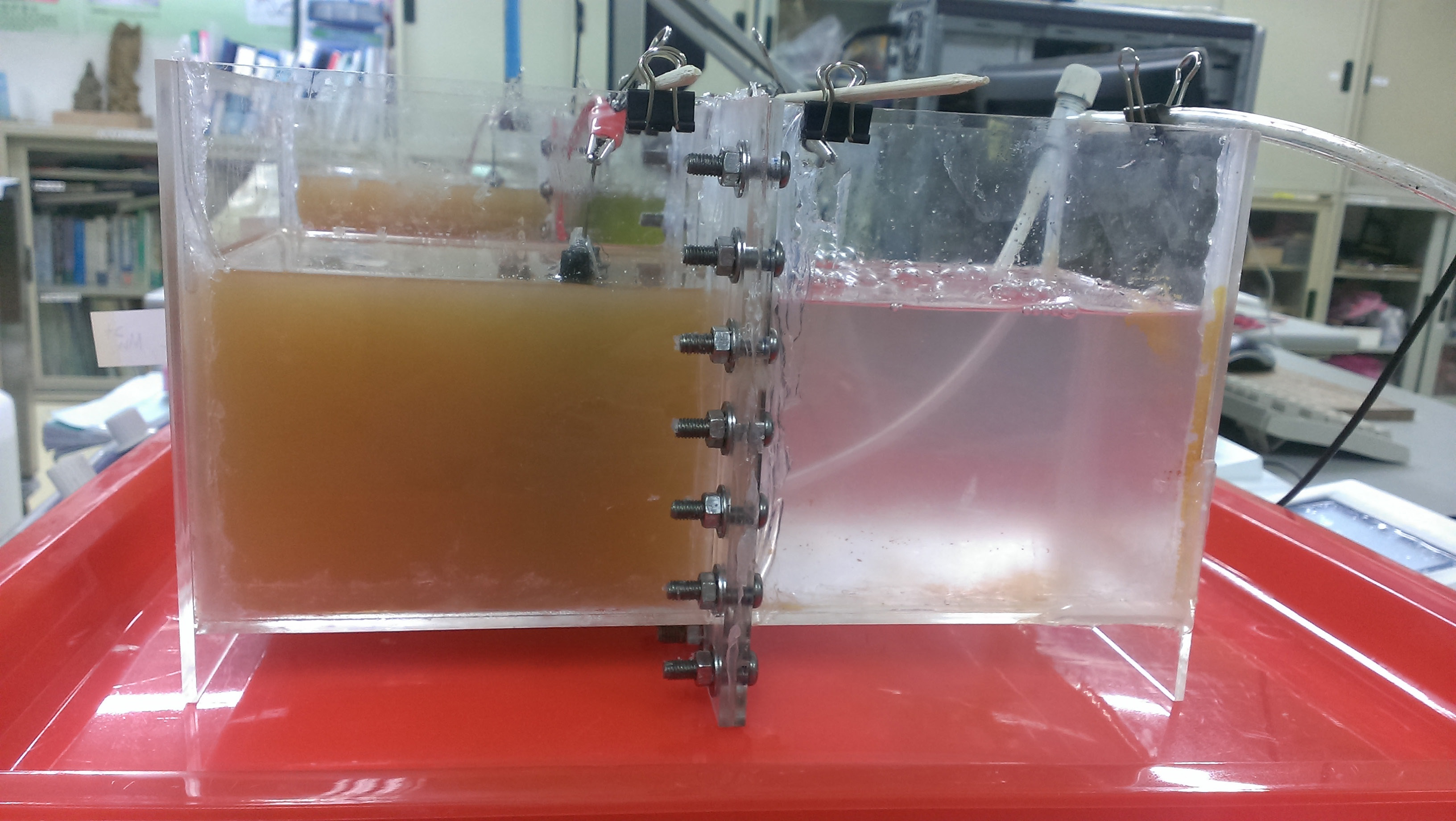 |
| (The left part is the anode chamber and the right part is the cathode chamber) Figure 1: Schematic diagram of dual-chamber BeF-MFC |
 |
| Figure 2: Variation of cell voltage within BeF-MFC with Fe2+ or Mn2+ at charging condition of external resistance with 1.0 x 103 Ω |
 |
| Figure 3: Power performance shown as case of BeF-MFC with Fe2+ catalyst |
 |
| Figure 4: Power performance shown at case of BeF-MFC with Mn2+ catalyst |
 |
| Figure 5: Performance of cathode reduction shown in the case of BeF-MFC with Mn2+ catalyst |
| Electro-Fenton type | Anode | Cathode | Impact factor | Efficacy | Literature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chamber | Material | Solution | Material | Concentration of solution | Catalyst | Catalyst ratio | stir | Electron resource | pH | Glyphosate removal | COD removed | TOC/hr | Paper | |
| Electrolytic | SC | Pt | No | CF | 4-Chloro-2-Methylphenol 0.6 mM |
Fe2+ |
4 | Yes | 0.55V | 2.7 | No | No | 100%/6 hr | [17] |
| Electrolytic | SC | Pt | No | CF | Glyphosate 0.1 mM |
Mn2+ | 1 | Yes | Extra 200mA | 3 | 92%/0.7 hr |
No | No | [16] |
| MFC | DC | CF | Dairy Waste water |
CF | Oily Waste Water 3.7 mM | Fe2+ Mn2+ |
0.2 | Yes | Microbial | 3 | No | 40%/4 hr No effect |
No
|
This study |
SC: single chamber; DC: dual chamber; CF: Carbon Felt |
||||||||||||||






































