Top Links
Journal of Anesthesia and Patient Care
ISSN: 2456-5490
Capnography Detects Phrenic Nerve Stimulation during Cryothermal Ablation
Copyright: © 2016 O’Neill DK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
A 54 year old female presented for atrial fibrillation (AF) catheter cryoablation. She had experienced multiple pre-syncopal episodes prior to diagnosis and underwent a cardiac work-up including Holter monitor, transthoracic echocardiogram,stress test and tilt table test which was negative except for atrial fibrillation. She presented to the hospital for an AF ablation procedure under general anesthesia after transesophageal echocardiogram to rule out atrial thrombosis formation.
The patient was brought to the cardiac electrophysiology laboratory with a 20 gauge intravenous line in place in the left antecubital fossa. Standard ASA monitors and a BIS monitor were placed prior to induction. After induction of anesthesia, with propofol 250 mcg/kg/min x 5 min, remifentanil 0.05 mcg/kg/min x 5 min, fentanyl 250 mcg, rocuronium 50 mg, intubation was performed and placement was confirmed with end-tidal carbon dioxide (ETCO2). An esophageal temperature probe and a gauze bite block were also placed. Anesthesia was maintained with remifentanil and propofol infusions for duration of the procedure. The depth of sedation with propofol was titrated to maintain a BIS of 45-55 throughout. Neuromuscular blockade was not maintained after induction to allow phrenic nerve monitoring during phrenic nerve pacing with a 3.5-mm-tip catheter (ThermoCool NaviStar, Biosense Webster) to determine ablation catheter misplacement.
At the time of cryoablation and phrenic nerve monitoring, the capnogram demonstrated interruption of normal exhalation (Figure 1) consistent with diaphragmatic movement and preservation of phrenic nerve integrity. The ETCO2 capnogram demonstrated the classic “curare cleft” and the flow sensor triggered a pressure support breaths demonstrated by vertical line in flow and pressure waves. There was a decrease in EtCO2 and change in slope of the capnogram. The cooling from the cryoablation was also noted with a 7-10 degree Centigrade drop in measured temperature at the tip of the esophageal probe.
The case proceeded uneventfully and the patient was extubated and taken to PACU without incident.
Atrial fibrillation is the most common arrhythmia affecting more than 5 million Americans.1 Treatment options have focused on controlling the patient’s heart rate or rhythm. However, many pharmacological therapies are ineffective or intolerable due to side effects. Ablation therapy, which is targeted myocyte necrosis, has been widely used to treat this condition. Ablation is most frequently performed via heating the tissue with radiofrequency energy. Alternatively, ablation can be performed by freezing tissue, typically called cryoablation. Ablation therapy for atrial fibrillation is typically aimed at the myocytes located within pulmonary veins where AF substrate and foci most commonly arise.
The right phrenic nerve runs between the SVC posteriorly and the right upper pulmonary vein; it then proceeds anterolaterally to the RA/SVC junction (Figure 2). Phrenic nerve pacing with observation of diaphragmatic movement is frequently performed to assure that the heated or cooled catheter is not injuring the phrenic nerve during ablations of the SVC or right upper pulmonary vein (RUPV), atrial tachycardia and epicardial ventricular tachycardia ablations, or biventricular ICD placements. The phrenic nerve can be damaged while ablation therapy is performed due to the proximity of the phrenic nerve to the AF ablation energy source. 3 When ablation is performed, the right phrenic nerve is frequently stimulated with high output pacing from the SVC in order to monitor function of this nerve. Ablation is terminated if phrenic nerve stimulation of diaphragmatic movement becomes attenuated. Cryoablation is different from RF ablation in that, the loss of electrical activity is reversible at -30 degrees centigrade. When right sided cryoablation is performed, the catheter is cooled and diaphragmatic stimulation is performed via the ipsilateral phrenic nerve. If diaphragmatic movement is attenuated despite phrenic nerve stimulation proximal to the ablation location, cooling is discontinued and phrenic nerve function typically returns (Figure 3).
Anesthetic considerations include the monitoring the integrity of neuromuscular junction function while phrenic nerve stimulation is being performed. In each of these scenarios, continued neuromuscular blockade would obviate the usefulness of phrenic nerve monitoring and increase the risk of phrenic nerve injury from the procedure. These patients are usually under general anesthesia or deep sedation in order to tolerate mapping and ablation of AF. It is critical that patients have intact nerve function and therefore, do not have muscle relaxant effects during phrenic nerve stimulation. As anesthesiologists who often work outside of the operating room, we need to be familiar with these emerging therapies. Due to our physical separation from the operating room, there is a great chance that we may not have trained personnel in our immediate vicinity that can differentiate true anesthetic emergencies from anticipated complications.
It is important to recognize that diaphragmatic movement will be detected in the capnograph and this does not signify light anesthesia and/or voluntary patient movement. Therefore, the phrenic nerve stimulation of the diaphragm should not be treated with neuromuscular blockade in these cases. While phrenic nerve injury typically resolves, the injury frequently persists for up to one year and may not always resolve. The elevated hemidiaphragm frequently leads to dyspnea on exertion.
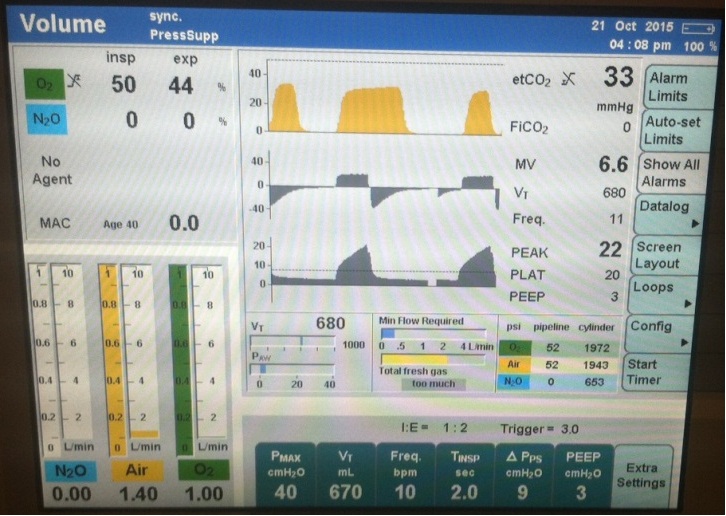 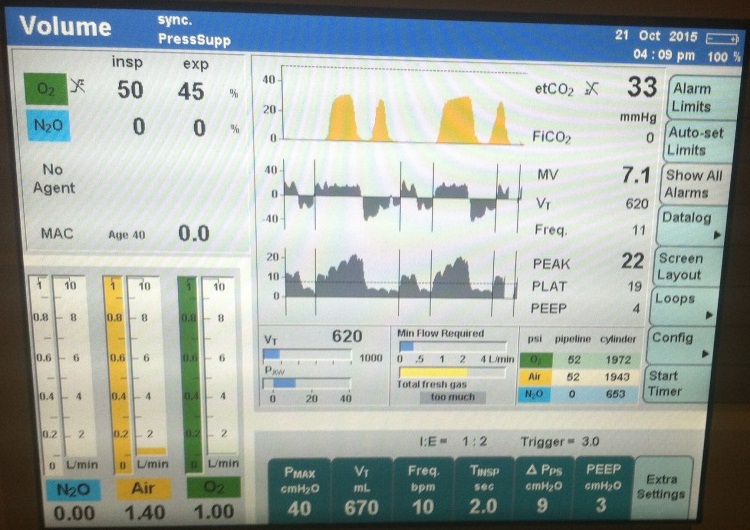 |
The ETCO2 capnogram demonstrated the classic “curare cleft” and the flow sensor triggered a pressure support breaths demonstrated by vertical line in flow and pressure waves |
 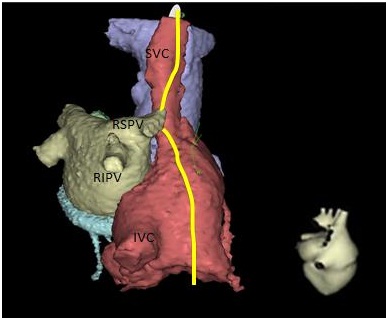 |
| A right posterior oblique view of the right atrium (red), left atrium (gray), aorta (blue) and coronary sinus (green). The right phrenic nerve (yellow) descends along the right anterior-lateral border of the superior vena cava (SVS). Along its course, the nerve curves posteriorly around the SVC towards the right pulmonary veins, closest to the right superior pulmonary vein (RSPV), but also within proximity of the right inferior pulmonary vein (RIPV). The nerve then proceed along the anterior-lateral right atrium Figure 2: Typical Course of Right Phrenic Nerve |
Figure 3: Algorithm for Phrenic Nerve Pacing |






































