Top Links
Journal of Veterinary Science and Animal Husbandry
ISSN: 2348-9790
Preliminary Investigation of the Interaction of Misoprostol and Phenylbutazone on Bone Response to Injury in Horses
Copyright: © 2018 Anderson DE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Phenylbutazone (PBZ) is commonly used in equine patients for treatment of orthopedic injuries. Phenylbutazone may adversely affect bone healing because of suppression of prostaglandin production. We hypothesized that administration of the prostaglandin analog misoprostol would enhance bone healing and mitigate the untoward effects of PBZ on bone response to injury in horses. The objectives of this study were to determine whether the administration of misoprostol would enhance bone healing and whether concurrent administration of PBZ and misoprostol would mitigate the untoward effects of phenylbutazone. Twenty horses were randomly assigned to one of four groups (n=5 per group): Group 1 (untreated control), Group 2 (phenylbutazone alone), Group 3 (misoprostol, alone), or Group 4 (concurrent treatment with phenylbutazone and misoprostol). A 4.5-mm diameter uni-cortical bone defect was created in one metacarpal III bone of all horses. Fluorochromic bone labels were administered intravenously on Days 0, 7, and 14. Computed tomographic osteoabsorptiometry and histomorphometric analyses were performed on the harvested metacarpal bones. Phenylbutazone treatment caused a decrease in endosteal new bone formation. Administration of misoprostol appeared to mitigate the magnitude of the PBZ effect on new bone formation (endosteal in-growth, p<0.06). Bone specific alkaline phosphatase serum activity decreased throughout the 14-day period of stall confinement. Mineral apposition rates increased in all groups during the period from 7 to 14 days after bone injury. Further research is needed to determine if this effect is significant. The administration of misoprostol may be beneficial to lessen the undesired impact of phenylbutazone on bone healing in horses.
Keywords: Phenylbutazone; Bone; Misoprostol; Horses; Prostaglandins
Phenylbutazone (PBZ), a non-selective cyclooxygenase inhibitor, is a commonly used non-steroidal anti-inflammatory drug (NSAID) in the treatment of horses suffering orthopedic disease or injury [1]. However, experimental studies performed on animals have documented inhibitory effects of NSAIDs on bone healing [3-9]. Research done in horses has demonstrated that PBZ causes a decrease in mineral apposition rates and appeared to decrease the healing rate of cortical defects in horses [10]. If this effect is pronounced, use of PBZ during the early post-injury period may be counterproductive due to the potential to adversely affect the biological response of bone to trauma.
Prostaglandins have been shown to be important mediators in the early phases of bone healing in rat and rabbit models [3-9]. Bone trauma stimulates a local increase of prostaglandin concentrations, which stimulates proliferation of osteoprogenitor cells during bone healing. Prostaglandin E2 (PGE-2) stimulation of cell activity and recruitment of undifferentiated stem cells as progenitor cells is mediated by prostaglandin interaction with E-prostanol receptors (EP1, EP2, EP3, and EP4) [11-20]. Selective inhibition of EP4 receptors has a significant inhibition of cell differentiation in vivo; therefore, activation of this receptor likely has a significant impact on new bone formation. Administering PGE-2 in a rodent model yielded increased cortical and cancellous bone mass and increased the mechanical strength of the bone [15]. In the same way, research in rodents have shown that prostaglandin E1 (PGE-1) activates both EP2 and EP4 receptors, which stimulates new bone formation [13-20]. Both PGE-1 and PGE-2 have similar potency with respect to their activation of EP4 receptors. Therefore, it is expected that PGE-1 and PGE-2 would have similar potency with respect to stimulating new bone formation. A significant reduction in the production of PGE-2 has been documented in horses administered PBZ [21].
We hypothesized that supplementation of prostaglandins will enhance bone healing and may be a useful adjunct in management of equine orthopedic injuries. The concurrent administration of selected PGE analogs with PBZ may negate or reverse the down regulation associated with phenylbutazone administration alone. Misoprostol is a commercially available PGE analogue that is available as an oral tablet. It may offer an option to either stimulate bone healing or to negate the inhibition of bone healing activity with PBZ therapy. We hypothesized that administration of misoprostol will mitigate the inhibitory effects of PBZ on bone activity. The objectives of this study were to determine: a) whether administration of misoprostol can improve bone response to bone injury using a unicortical defect model, and b) whether administration of misoprostol can mitigate the inhibitory effects of PBZ on bone activity.
Twenty healthy, mature horses were purchased for use in this study in accordance with an Institutional Animal Care and Use Committee (IACUC Protocol Number: 2003A0070) approved protocol. Horses enrolled in this study included Standardbred (n=14), Saddlebred (n=3), Warmblood (n=2), and a Palomino horse. Horses were a mean age of 10 +/- 6.7 years and weighed a mean of 478 +/- 48 kgs. All horses were acclimatized and kept in individual stalls (3-m x 5-m), beginning 2 weeks prior to the start of the study. The horses received hay and water ad libitum and 1 kilogram (kg) of mixed grain with molasses twice daily. Prior to entry into and for the duration of this study, all horses had daily physical examination and evaluation for lameness at a walk. Lameness was assessed by thorough inspection and palpation of the limb and visual observation of gait while being led by halter and lead rope in a straight line and during turns. Each horse was weighed at the start and the end of the study period. Horses were randomly assigned, using a Latin Square Block Design, into one of 4 treatment groups (5 horses each): Group 1 (control), Group 2 (phenylbutazone; PBZ), Group 3 (misoprostol; M), and Group 4 (phenylbutazone and misoprostol; PBZ+M). In order to eliminate influences of left vs right limb effects, a single left or right third metacarpal bone was randomly assigned for creation of a cortical defect in each horse. Phenylbutazonea was orally administered to Groups 2 and 4 at 4.4 milligram (mg)/kg twice daily for 14 days. This dose was based on previous work with this model where the effect of this dose of PBZ had been validated [10]. A synthetic prostaglandin E analogue, misoprostolb was administered orally to Groups 3 and 4. As no data was available for the bioavailability and pharmacokinetics of misoprostol in the horse, the dose of misoprostol administered was extrapolated from clinical reports where a clinical effect had been observed. We desired to maintain a relatively frequent dosing interval in an attempt to maintain consistent exposure to the drug. These combined startegies resulted in the selection of a dose of 2 μg /kg body weight give orally twice daily for 14 days.
Oxytetracycline hydrochloridec was administered (20 mg/kg, IV) immediately after surgery on Day 1 and again on Days 7 and 14 as a fluorochromic bone label. Serum for analysis of bone specific alkaline phosphatase activity was collected from all horses on Days 1, 7, and 14 to assess bone activity in vivo [15]. Each horse was placed in a set of standing stocks for catheter placement at the time of surgery and for the duration of the surgical procedure. Sedation was provided using xylazine hydrochloride (0.66 mg/kg, IV) and butorphanol tartrate (0.044 mg/kg, IV). Local anesthesia was provided using 2% lidocaine hydrochloride (6 mL) infused subcutaneously approximately 5 centimeters (cm) distal to the proximal metacarpus on the lateral aspect of the third metacarpus at the site of the anticipated location of the skin incision.
The skin overlying the third metacarpal bone was clipped and aseptically prepared for surgery. Using a #10 surgical blade, a 2 cm long incision was made in the frontal plane, parallel to and located approximately midway along the length of the metacarpal bone. A 4.5-mm defect was created by aiming the drill bit perpendicular to the surface of the cis-cortex, through the skin incision. The skin was closed using a standard skin stapler. A light bandage was applied to cover the skin incision, using a sterile non-adhesive dressing material, a non-sterile stretch bandage, and elastic tape. The bandages were changed daily and the horses monitored for incisional swelling, discharge, or pain. Pain was assessed by response to manual palpation of the limb and assessment of lameness by the same investigator (KDN). At each bandage change the horses were evaluated visually for lameness at the walk for a minimum of 5 minutes. All horses were humanely euthanized on Day 15 using a lethal injection of euthanasia solution.d The diaphysis of the third metacarpal bone was harvested at completion of the study. The overlying soft tissues were removed, and the metacarpal bone specimens were harvested centered on the bone defect, and stored at -70 oC until analyzed.
a Bute Tabs, 1 g, Vedco, Inc., St. Joseph, MO
b Cytotec, 200 μg, Pharmacia Corp., Chicago, IL
c OxyCure 200, 200mg/mL, Vedco Inc., St. Joseph, MO
d Euthansol, Virbac AH Inc., Fort Worth, TX 76161
Each bone specimen was imaged together with a calibration phantom in air by use of a CT scanner. e A solid dipotassium phosphate calibration phantomf was used for internal calibration of each specimen scanned. The dipotassium phosphate phantom was composed of a plastic base material containing 5 rods of reference material embedded in the plastic base. The reference materials contained known and varying amounts of low and high atomic number materials. A series of 1-mm transverse contiguous slices were acquired from 1 cm proximal to 1 cm distal to the drill site. Exposures were made at settings of 125 mA, 130 kVp, and 2 seconds. A bone algorithm format was used with a small field of view of 14-cm. Images were constructed on a 512 x 512 matrix with a voxel size of 0.27 mm x 0.27 mm x 1.0 mm. After imaging was completed, the metacarpal specimens were returned to the -70 °C freezer until sectioned for histomorphometric analysis.
Hounsfield density values, as determined from CT, were converted to dipotassium phosphate equivalent density (PPED) values using the following equations: PK2HPO (unknown) = (μROI – ßCT) / ÕCT and μROI - P water = (PK2HPO4)(Õ ref) + ß ref , where: μROI = CT number within a region of interest (ROI) in a reference or unknown material in Hounsfield Unit (HU), PK2HPO4 = K2HPO4 equivalent density of material within the measured ROI, P water = water equivalent density of material within the measured ROI, Õ ref = imaging technique-specific parameter defining the response of the CT scanner to K2HPO4, and ß ref = imaging technique-specific parameter characteristic of the CT number scale. Õ ref and ß ref are solved for using a nonlinear regression model: ÕCT= Õ ref – 0.2174 and ßCT = ß ref + 999.6.g
A standardized, elliptical region of interest (ROI) was drawn encompassing the bone defect within each specimen. The size, shape, and position of the ellipse were unchanged for each specimen. The HU values of each ellipse were recorded, beginning on the slice before, and ending on the slice after the drill defect. For each specimen, the drill defect, as observed on multiple slices, was hand traced and the corresponding ROIs and HU values recorded. One hundred PPED-interval scatter plots of the CT slice with the largest drill defect ROI were created to improve discrimination of the bone density distribution within each drill defect.
After completion of CT, the metacarpal specimens were trimmed to within 1 cm of the unicortical defect. Samples were dehydrated using ethyl alcohol and xylene washes, and embedded in polymethylmethacrylate: a polymer of 75% methylmethacrylate, 25% dibutyl phthalate, and 2.5% benzoyl peroxide. Two, 200 μm thick slices were taken tangentially through the unicortical defect using a calibrated Isometric saw with a diamond cutting wheel. One slice from each sample was groundh to a thickness of 50 μm. All 50 μm sections were left unstained, whereas all 200 μm slices were etched using 1% hydrochloric acid and acid alcohol and then stained with Zesiger Blue. The unstained, 50 μm thick sections mounted on the microscope slides were examined under brightfield and fluorescent lighting (450 nm).i
Mineral apposition rates (MAR) were calculated using triple labeled osteons present in each 50 μm section within 5-mm of the unicortical defect as previously described [10] (Figure 1). The distance between fluorochromic labels was measured using a calibrated ocular ruler and the 40x objective and averaging four equidistant measurements between three adjacent fluorescent labels. The distance corresponding to the first interval (Days 1-7) between the outer and middle rings was measured and recorded. The distance corresponding to the second interval (Days 8-14) between the middle and inner rings was measured and recorded. The mineral apposition rate was calculated by dividing the distanced measured between rings by the number of days corresponding to the interval between administrations of the fluorochromic labels.
ePicker PQS CT scanner, Phillips Medical Systems, Bothell, WA.
fCann-Genant Model 3 CT, Quantitative Technologies, South San Francisco, CA
gSigmaPlot 8.02, SPSS, Inc., UK.
The histologic score for the bone reaction for each bone specimen using the 200 μm section and the 2x objective was recorded. The periosteal reaction was scored using the calibrated ocular ruler and measuring the extent of the periosteal reaction originating at the defect periosteal junction and along the surface of the cortex as previously described [10]. The endosteal reaction was graded subjectively as absent = N, partial (without bridging of the defect) = P, and bridging = B. The percent endosteal in-growth of the defect was calculated using a calibrated ocular ruler and measuring the extent invasion of the defect from the endosteal surface.
Bone specific alkaline phosphatase activity was measured with a commercially available enzyme linked kitj validated for use in horses. Optical reading was performed at 405 nanometers using an automatic plate reader.k The standards included in the kit were used to generate a quadratic calibration curve to enable conversion of the optical readings to U/L.
Analysis of variance (ANOVA) with repeated measures was used to determine if interactions existed between the different treatment groups.I The dipotassium phosphate equivalent density values, percent defect filling, and mineral apposition rates were analyzed using a one-way ANOVA. The endosteal response and endosteal in-growth of the defect were analyzed using a Kruskal-Wallis test. Osteonal activity, bone specific alkaline phosphatase activity, and mineral apposition rates were analyzed using a two-way analysis of variance (ANOVA) with repeated measures. Post-tests included Turkey (paired and unpaired t-test, and one-way ANOVA), and Bonferroni (two-way ANOVA) to determine whether differences were statistically significant. A P value of < 0.05 was considered significant; a P-value of < 0.1 was considered a significant trend.
The unicortical metacarpal defect was successfully created in all horses. No clinical signs of lameness or morbidities were observed in any horses following the creation of the defect. Clinical signs of drug associated morbidities were not observed in any of the horses that received phenylbutazone or misoprostol.
Significantly fewer PBZ treated horses (Group 2) had endosteal in-growth into the bone defect compared with horses assigned to the control group (p< 0.03) and trended to have fewer as compared to misoprostol (p<0.06), and PBZ+misoprostol (p<0.06) groups (Figure 2). Endosteal ingrowth was similar among control, misoprostol, and PBZ+misoprostol treated horses (p=0.4; Figure 4).
hMurato 521, Murato Co Ltd., Tokyo, Japan
iOlympus Optical Company Ltd., Japan
jMetra BAP EIA Kit, Quidel Corp, San Diego, CA
kAscent Software Version 2.6, Thermo Lab Systems. Beverly, MA
lGraphPad Prism 4.0 version 4.01 for Windows, GraphPad Software, San Diego CA
There was no significant (P > 0.05) difference in the MAR between treatment groups (two-way ANOVA). However, time was a confounding factor having a significant (P < 0.0014) effect on the MAR. Horses assigned to all groups had increased MAR during the period between day 7 and 14 as compared with the period between day 1 and 7 (Figure 3). In all horses, serum alkaline phosphatase activity significantly decreased during the study period (p< 0.01; Figure 5). There were no significant differences in bone specific alkaline phosphatase activity among treatment groups. The mean and SD for bone specific ALP in all horses was significantly greater on Day 1 (53.33 ± 18.86 U/L) compared with Day 7 (49.11 ± 20.78 U/L) and Day 14 (42.30 ± 16.99 U/L).
The osteonal density was similar for all treatment groups (control: 20.61 ± 3.54 osteons/mm2; PBZ: 21.15 ± 3.14 osteons/mm2 ; M: 23.04 ± 2.81 osteons/mm2; PBZ+M: 22.39 ± 3.14 osteons/mm2. There was no significant (P > 0.05) difference in the osteonal density between treatment groups (one-way ANOVA).
CT image analysis was completed in all horses except for 1. In one metacarpal specimen from Group 3 (M), an erroneous PPED value prevented interpretation of density and was excluded from statistical analysis. The mean bone density (PPED ± SD) for Group 1 (C) was 246.8 ± 83.2, Group 2 (PBZ) was 351.2 ± 152.1, Group 3 (M) was 288.4 ± 98.9, and Group 4 (PBZ+M) was 258.1 ± 107.6. There was no significant (P > 0.05) difference between treatment groups for bone density (one-way ANOVA).
Based on the results of this preliminary study, misoprostol may have value to mitigate the inhibitory effect of PBZ on bone activity and formation in horses. As expected, endosteal new bone formation was markedly less in PBZ treated horses as compared to untreated controls. Although the group sizes were small, horses treated with misoprostol, with and without concurrent treatment with PBZ, had more bone formation (trend) as compared with horses treated with PBZ alone; the p-value (p<0.06) approached significance. Treatment group sizes were small and likely have limited the interpretation of the results. The PGE analog drug, misoprostol, appeared to have a positive effect on bone healing and seemed to mitigate the negative effect of PBZ on bone response to injury. An interesting observation in this study was the endosteal responses to acute injury. Early healing of the defect was characterized by endosteal in-growth into the defect. Recruitment of endosteal osteoprogenitor cells appears to be a necessary prerequisite of the early phases of bone healing in this model of bone injury in horses. A major limitation of the study was selection of a dose and dosing frequency for misoprostol. There is limited information on the use of this drug in horses and pharmacokinetic studies are needed to help guide clinical decision making. We chose a dose based on the best available information and constraints present at the time. This preliminary study may support future research investigating the effects of misoprostol on bone activity in horses, but the pharmacodynamics of this drug needs to be fully elucidated prior to planning such studies.
Phenylbutazone, when given alone, appeared to decrease bone healing in response to injury as measured by the endosteal response. Phenylbutazone previously has been shown to decrease the mineral apposition rate and reduce the mineralized tissue filling bone defects using this horse model [10]. Endosteal ingrowth was significantly less in PBZ horses as compared with control, misoprostol, and misoprostol-PBZ treated horses. Although variances within the groups likely prevented small differences being detected between the treatment groups, misoprostol co-treatment with PBZ appeared to have the potential to mitigate the inhibitory effects of PBZ.
The bone specific alkaline phosphatase activity declined in all horses during the study period, and is consistent with that previously reported in horses during stall rest [22]. The bone specific alkaline phosphatase activity indicates a systemic decrease in metabolic activity, including decreased matrix production and mineralization [23-25]. Horses administered misoprostol (Groups 3 and 4) had a trend towards decreased activity similar to the controls during the first 7 day period; however, during the second 7 day period Groups 3 and 4 more closely resembled the horses only administered phenylbutazone (Group 2). Further research, perhaps utilizing greater doses or more frequent administration of misoprostol would be needed to explore this question.
Our assumption was that misoprostol would have similar pharmacodynamics in horses as it does in other monogastric species [26-29]. PGE activates both EP2 and EP4 receptors to stimulate new bone formation [13-20]. Since both PGE-1 and PGE-2 have similar potency with respect to their activation of EP4 receptors, it was expected that that PGE-1 and PGE-2 would have similar potency with respect to stimulating new bone formation. Pharmacokinetics and pharmacodynamics for misoprostol for administration to horses had yet to be determined. We extrapolated the dose from previous research in other species and anecdotal reports of its use in clinical practice. Clinical dosing regimens for cervical relaxation or gastric ulcer therapy in horses vary. Although no pharmacokinetics studies have been published to date, the dosage of misoprostol, given orally to horses, has been reported at 5 ug/kg given once or twice daily. We chose to use a dose of 2 ug/kg q12hr for 14 days resulting in a total daily dose of 4 ug/kg [30,31]. We may have found a more profound effect with greater dosages but tablet formulation and economic constraints limited the study to exploration of a single dose. Other potential confounding factors affecting the oral bioavailability of misoprostol in humans include ingesting food when administering the misoprostol and the concurrent administration of antacids [29]. In this study, all drugs were administered with feed. It is unknown whether administering misoprostol with feed limited the bioavailability in the horse. A pharmacokinetic study in the horse is required to elucidate the bioavailability of misoprostol in this species. This could explain why misoprostol did not have a significant effect on osteonal activity and the mineral apposition rates. Drug interactions between misoprostol and NSAIDs have not been observed in humans and laboratory experimental animals. Further research regarding oral bioavailability and pharmacokinetics of misoprostol in horses is needed to provide a basis for planning future studies. Large variances in our dataset likely prevented small differences from being detected among the treatment groups.
Direct methods to assess bone metabolic activity and healing include the use of fluorochromic markers and histomorphometry, including osteonal activity and mineral apposition rate [22]. Large variances within the treatment groups reduced the ability to detect small differences in MAR between groups. Histomorphometry showed significant decrease in new bone formation in horses receiving PBZ, alone.
Computed tomographic (CT) osteoabsorptiometry was useful to identify and measure the defect area. There was no correlation between the PPED values and the endosteal filling of the defect for each specimen. The early phases of bone healing is characterized by the presence of new bone formation comprised of woven bone, which appears histologically quite porous compared to dense cortical bone. The findings of the present study suggest that CT likely has low utility in identifying new bone formation within a defect during the early phases of bone healing. Narrowing the PPED-interval scatter plot range may have increased the sensitivity in identifying patterns of density distribution more characteristic of early bone healing.
Regional acceleratory phenomenon (RAP) has been previously described, including horses, where a local increase in the mineral apposition rates adjacent to a defect was observed [32,33]. The localized enhancement of both soft and hard tissue healing processes is characteristic of RAP. This phenomenon promotes faster healing by increasing the local blood supply and osteoblast recruitment [32]. New bone formation is characterized by the presence of woven bone, an indicator of RAP. Furthermore, it has been proposed that prostaglandins may initiate RAP [32].
Based on the results of this study, prostaglandin analogs may have the potential to be used to mitigate the inhibitory effects of PBZ on bone healing in horses. Early bone healing was characterized by the in-growth of the defect by the bridging endosteal response. Phenylbutazone significantly decreased the endosteal response when administered without misoprostol. The early and short study period likely limited the ability to study the interactions between phenylbutazone and misoprostol therapy on bone density and mineralization rate of cortical bone. Time was a confounding variable with respect to the mineral apposition rates and bone specific alkaline phosphatase activity. The misoprostol dose and frequency in the horse has yet to be determined; therefore, a pharmacokinetic study is required since there is some evidence that misoprostol may be protective during the first 7 days of bone repair. Further research using misoprostol may be valuable to determine the effects of prostaglandin E replacement therapy on bone activity and mineralization rate of cortical bone in horses. Furthermore, a longer study period would likely facilitate detecting differences between treatment groups.
This study was conducted as part of a Master’s of Science (Newman) degree at the College of Veterinary Medicine at The Ohio State University. Funding for the project was provided by Equine Racing Funds, College of Veterinary Medicine, The Ohio State University, and Columbus, OH 43210. The authors also wish to thank Dr. J. Lakritz and Dr. R. Sams for technical assistance and support; Orthopedic Pathology Laboratory; and Johnna Johnson, Dan Linden, Paul Ramsey and Cara Levine for assistance.
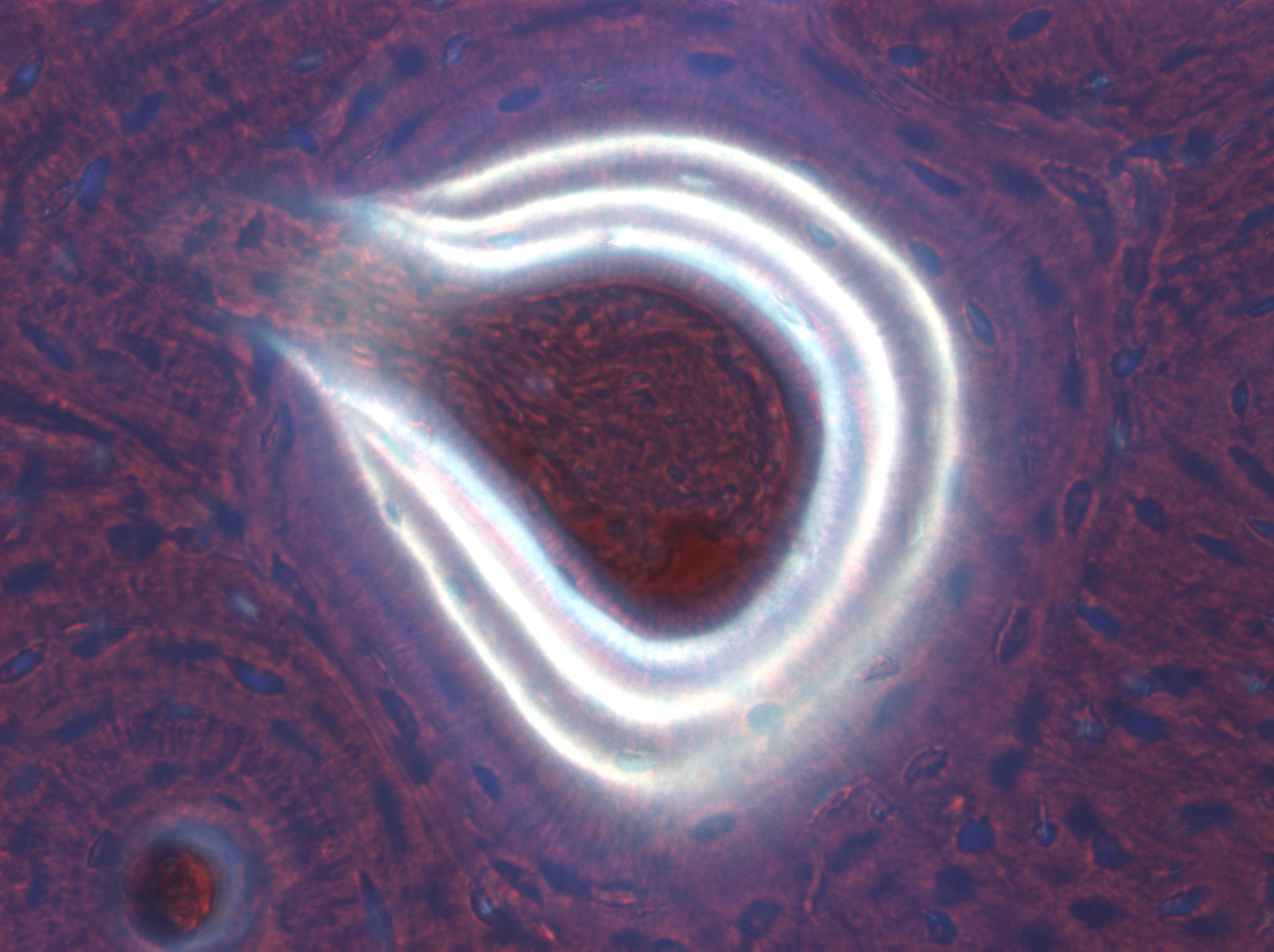 |
| Figure 1: Image of triple labeled osteon in an undecalcified metacarpal bone specimen (50 �m thick section; fluorescent light at 450 nm; 20x objective). The three rings correspond to administration of oxytetracycline (30 mg/kg) IV on Days 1 (outer ring), 7 (middle ring), and 14 (inner ring). |
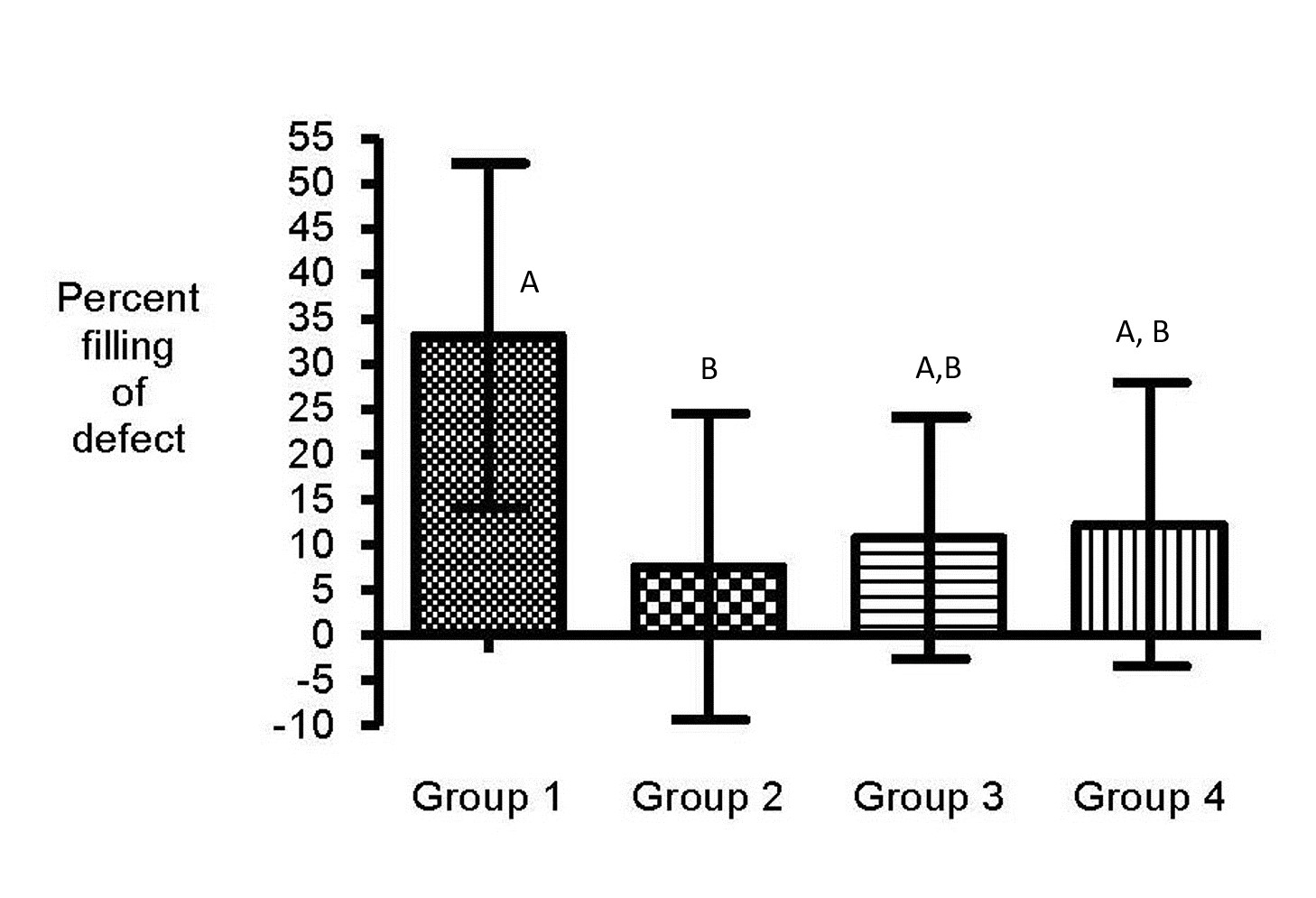 |
| Figure 2: Histology slide depicting endosteal in-growth into the defect (groups with different letters are significantly different). In-gorwth was significantly less in PBZ treated horses as compared with control horses (p< 0.03) and trended to have less than misoprostol (p<0.06) and PBZ+misoprostol (p<0.06) treated horses. Endosteal ingrowth was similar among control, misoprostol, and PBZ+misoprostol treated horses (p=0.4) |
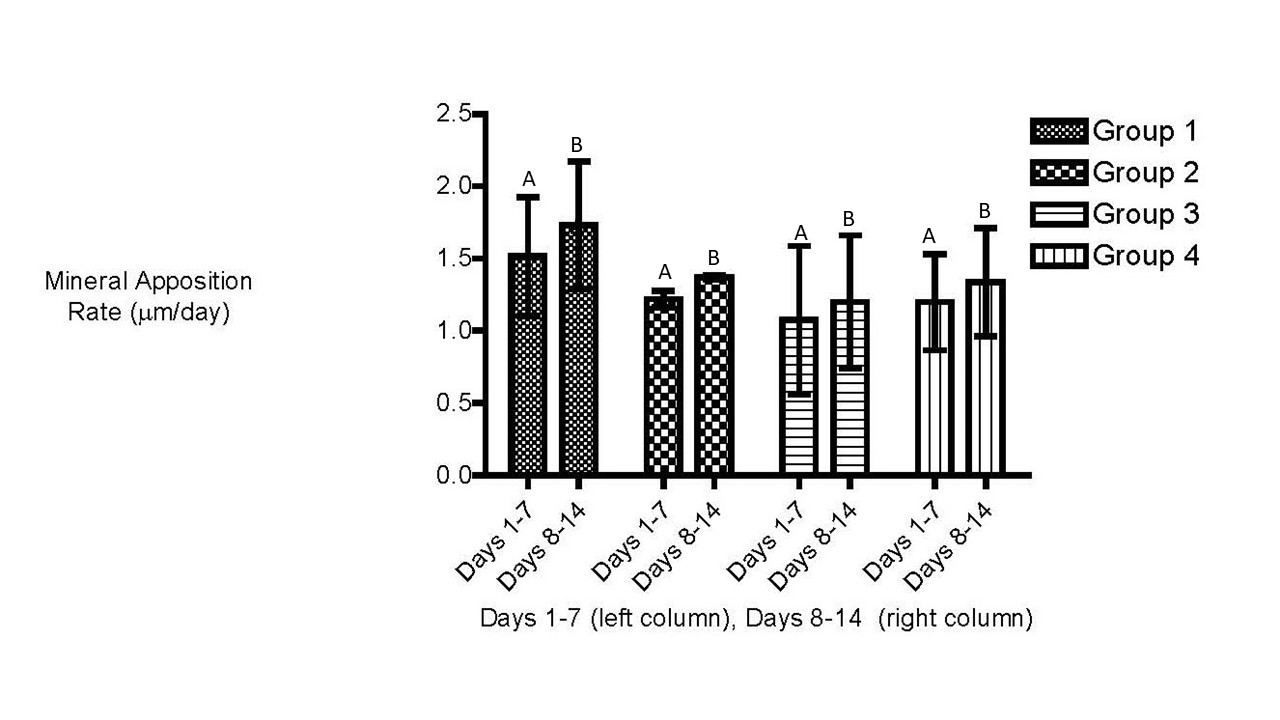 |
| Figure 3: Mean mineral apposition rate (MAR) �m/day � SD. There were no significant (P > 0.05) difference in the MAR between treatment groups (two-way ANOVA). Horses assigned to all groups had increased MAR during the period between day 7 and 14 as compared with the period between day 1 and 7. Figures with different letter designations are significantly different. |
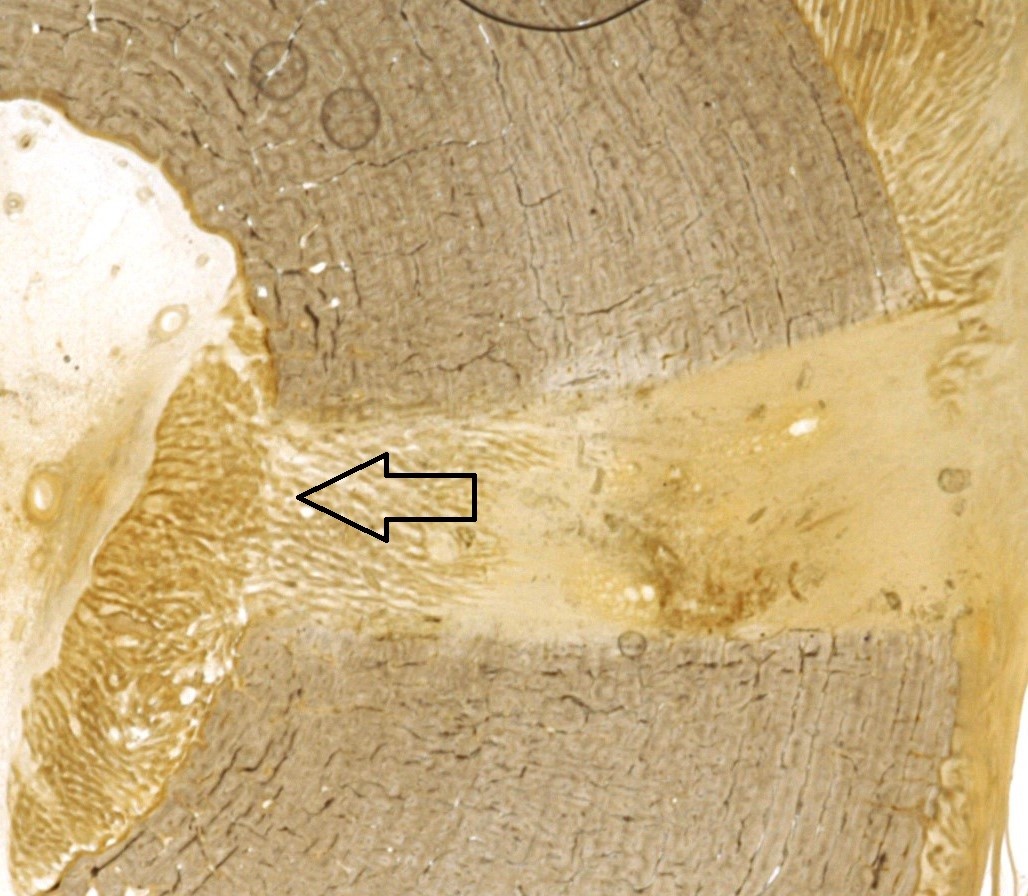 |
| Figure 4: Histology slide of a representative bone specimen used in histomorphometry analysis. New bone formation can be seen originating from the endosteal surface (arrows). |
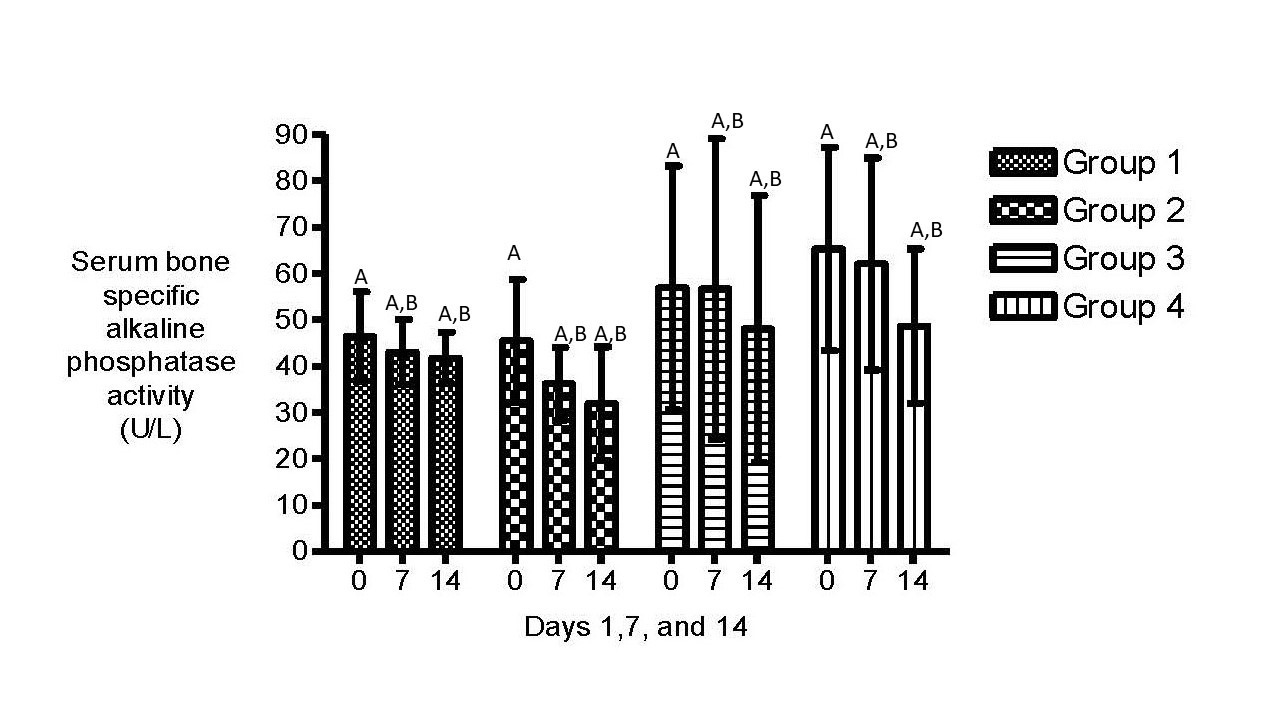 |
| Figure 5: Percentage decrease of bone-specific alkaline phosphatase activity for all horses from baseline (Day 1). Serum alkaline phosphatase activity significantly decreased during the study period with groups (p< 0.01; Figure 5). There were as no significant differences in bone specific alkaline phosphatase activity between treatment groups (A); ALP activity declined in all horses over time (B). |






































