Top Links
Journal of Veterinary Science and Animal Husbandry
ISSN: 2348-9790
Assessment of Blood Concentration of Cardiac Troponin I (cTnI) in Healthy Calves and Stocker Calves Effected with Bovine Respiratory Disease Complex
Copyright: © 2017 Anderson DE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Bovine respiratory disease complex remains one of the most economically important diseases of stocker cattle. Reports of myocardial injury associated with BRDC have been limited to necropsy findings. An animal-side diagnostic test for cardiac troponin I (cTnI) has been validated for use in cattle. The objective of this study was to determine the association of blood concentration of cTnI and pneumonia in stocker cattle. A proof-of-concept study was conducted using 16 calves (7 healthy; 9 BRDC). Then, a clinical trial was conducted in commercial stocker cattle. Calves were randomly selected at arrival (n = 35) and during treatment for BRDC (n = 66) from a population of 293 stocker calves. In this trial, venous blood samples were obtained and placed in a heparin tube and immediately analyzed using an I-STAT 1 hand held blood analyzer. Statistical analysis included a Student’s T-test (proof-of-concept study) and generalized linear models procedures (clinical trial) to evaluate potential associations with cTnl and BRDC. In the first study, cTnI was significantly greater in calves having respiratory disease (0.04 +/- 0.04 ng/ml) when compared with calves not having respiratory disease (0.006 +/- 0.01 ng/ml; P = 0.036). During the clinical trial, blood concentration of cTn-I in calves at arrival (0.01 +/- 0.02 ng/ml) and in calves that remained healthy (0.01 +/- 0.02 ng/ml) were significantly less when compared with calves suffering BRDC at any time point (0.05 +/- 0.01 ng/ml; P = 0.04). Calves that survived BRDC tended to have a lower cTnI (0.03 +/- 0.05 ng/ml) compared with calves that died from BRDC (cTn I 0.10 +/- 0.14 ng/ml; P = 0.055). Based on the results of this study, cTnI may be useful in assessment of the severity of BRDC and may be useful in predicting calves that will suffer prolonged or fatal complications of BRDC.
Keywords: Bovine; Stocker; Pneumonia; Cardiac Troponin I; cTnI
Bovine respiratory disease complex (BRDC) remains one of the most detrimental diseases suffered by feedlot calves and is a common clinical problem in all sectors of the cattle industry. BRDC represents economic losses because of diminished body condition, carcass quality, and weight gain [1]. BRDC also creates calf welfare concerns which impact decisions made by the producer regarding treatment, euthanasia, or slaughter of severely affected calves. Although significant advances have been made in research regarding specific pathogens and the interaction among viral and bacterial pathogens, accurate diagnosis and prognosis for individual cattle affected by BRDC in the field remains challenging. Observation of clinical signs such as lethargy, increased respiratory rate, labored breathing, nasal discharge, decreased appetite, and rectal temperature are commonly used to diagnose BRDC in field settings. Research has estimated that these methods of clinical illness scoring have a relatively low sensitivity and specificity for diagnosis of BRDC in cattle [2,3]. A review of feedlot management research identified the lack of accurate diagnostic methods for identifying calves affected with BRDC as a critical need and called for the development of quantitative measures to augment current BRDC diagnostic techniques [4].
Objective animal side measurements may become important tools to differentiate BRDC and non-BRDC cattle, may be helpful to predict response to therapy in BRDC cases, and may be useful in identifying cattle unlikely to recover from BRDC. Although several research trials have compared health outcomes between multiple antimicrobials [5,6], literature evaluating health outcomes compared to objective physiologic measurements is sparse. Recent studies have evaluated changes in available objective physiologic measures (complete blood count, serum biochemistry, arterial blood gas) following induced Mannheimia haemolytica pneumonia [7-9]. Relatively few associations between these parameters and the progression of pneumonia in the calves were found. Work evaluating rectal temperature illustrated that while broad associations between rectal temperature at the time of treatment had some association with outcomes, it did not have highly discriminatory prognostic power [10]. This work highlights the need for additional research to identify diagnostic and prognostic tools for identification of calves that may suffer severe BRDC less likely to be successfully treated. Diagnostic tests facilitating rapid results that may aid disease classification of BRDC and that offer the ability to predict which calves are likely to recover and which calves are more likely to suffer long-term debilitating disease would help to lessen the use of antimicrobial drugs, feed and labor costs, and lessen the number of calves suffering prolonged therapy without benefit. Accurate and timely response to debilitating disease may improve calf welfare by lessening suffering and lessen the futile use of antibiotics. This may also lessen the risk of food contamination by drug residues, meat adulteration by injection site lesions, and improve animal handling efficiency.
Troponin is a contractile protein produced by cardiac and skeletal myocytes. Troponin is part of the tropomyosin complex involved in relaxation and contraction of the muscle. When relaxed, myosin active sites are not available to interact with actin. When muscle contracture is activated and calcium is released into the cell from the sarcoplasmic reticulum, the binding of calcium to troponin results in exposure of myosin to actin causing contraction of the muscle. As such, increases in serum concentration of troponin can be used as an indicator of muscle injury. Cardiac troponin I (cTnI) is a myofibril protein specific to cardiac myocytes and is critical to the regulation of contractility of the myocardium. Troponin I is a myofilament protein, which is vital to myocardial cell function. cTnI is specific to heart muscle and provides a clinical tool to assess myocardial injury. Injury to the heart causes rapid release of troponin into the blood stream from cytosolic stores. cTn I is rapidly eliminated from the blood and continued presence of cTn I indicates ongoing release either associated with damage or myocardial regeneration [11]. Cardiac troponin I (cTn I) has become a common test for myocardial injury in people and is used in veterinary medicine for similar purposes [12-20]. Troponin I assays are increasingly available in table-top or hand-held biochemistry analyzers [21]. This presents an opportunity for field testing of large groups of cattle with rapid results that can aid in disease classification and outcome discrimination. Recently, serum cTn I has been reported for cattle having a variety of cardiogenic and non-cardiogenic disease [21-27].
We hypothesized that cTn I would be useful in assessment of calves having a clinical diagnosis of BRDC and that this serum biomarker would prove useful in predicting the health outcomes (death or re-treatment) of calves having BRDC. Study objectives were accomplished through two independent research projects. The first study (Study I) compared blood cTn I concentration between two independent populations of calves: 1) clinically normal calves and 2) calves confirmed to have BRDC. The second trial (Study II) evaluated Troponin I assays in selected high-risk calves at arrival to a stocker facility and in selected calves at each treatment for BRDC during the first 42-days on-feed after arrival at the stocker facility.
All calves in these studies were handled in accordance with protocols approved by the Kansas State University Animal Care and Use Committee.
Blood samples were obtained from either the jugular or coccygeal vein of each calf enrolled in the two studies. The blood was placed in a heparin tube and immediately analyzed using an I-STAT 1 hand held blood analyzer. Troponin I assays were performed exactly as described by the manufacturer. The i-STAT cTnI analysis unit uses test cartridges with two-site ELISA technology. The antibodies are specific for cTnI and use reagents derived from bovine and caprine sources. The detection range is 0 to 50 ng/ml (ug/L) with a C.V. of < 8% and an analytical sensitivity of 0.02 ng/ml.
Sixteen calves, ranging in age from 4 to 6 months, were used for proof-of-concept testing of cTnI based on health status. Seven healthy calves (n = 7 serum samples) were selected from a pool of calves that had been acquired for use in an unrelated study. These calves were assigned to the “normal” group. These calves had been observed by veterinarians with cattle expertise for a period ranging from 30 to 60 days before inclusion in the present study and were considered healthy based on prior observation, physical examination, daily observation, and growth performance characteristics. The health status of the calves was assessed further on the day of sampling through physical examination including detailed thoracic auscultation. Although no necropsy examinations were done, all normal calves remained healthy and free of clinical signs of BRDC for a minimum of 30 days after samples had been obtained. Nine calves (n = 9 serum samples) were selected for this study based on a clinical diagnosis of pneumonia. All of these calves had a clinical diagnosis of acute BRDC on the day the blood sample was drawn and tested. Diagnosis of BRDC was made by a veterinarian with cattle expertise based on clinical signs and physical examination including abnormal thoracic auscultation, varying combinations of increased respiratory effort, exercise intolerance, nasal discharge, lethargy, coughing, and increased rectal temperature >40 oC (104.0 F). Inclusion criterion for BRDC calves was confirmation of the diagnosis of pneumonia without cardiac disease by necropsy examination by experienced veterinarians with extensive cattle experience including post-mortem examination. An estimate of the reliability of the iSTAT cTnI test for determination of cardiac troponin in calves was done by repeated measures of whole blood samples collected as described above. Serum samples were analyzed in triplicate and the coefficient of variation (CV) determined.
Troponin assays were conducted in a field trial using calves randomly selected from a group of 293 stocker calves on arrival to the stocker facility. Inclusion criterion for calves in Study II included 1) healthy group (n = 35) - randomly selected calves which were healthy on the day of arrival to the stocker facility; and 2) calves that suffered BRDC (n = 66) at some point during the feeding period after arrival. Serum samples were obtained for cTnI analysis, at the time of arrival, from 35 calves free of any clinical sign of BRDC. Subsequently, serum samples were obtained from 66 calves that suffered BRDC, of which 6 had been selected for sample collection as part of the initial healthy-on-arrival group. Male, mixed breed beef calves were procured from the Southeastern US and shipped by truck to the KSU Beef Stocker Unit in north central Kansas. The cattle arrived in three independent loads and initial processing procedures were the same for all three loads. At arrival, all calves received an individual ear tag and each animal was weighed. Calves in each load were sorted by weight and gonadal status (steers, bulls) and randomly allocated to one of eight pens. The resulting assignments produced 24 pens of 12 or 13 head balanced for weight and gonadal status.
On the first day after arrival, all calves received preventative health products (a bacterin containing Clostridium chauvoei, septicum, novyi, sordelli, tetani, haemolyticum, perfringens Types B, C and D, a modified-live 4-way viral respiratory vaccine, and an injectable antiparasiticide) and intact males were surgically castrated. Based on pen treatment assignment, pens of calves received metaphylaxis using either subcutaneous tilmicosin at 1.5 ml/100 lb (1 mg/kg) of body weight or subcutaneous tulathromycin at 1.1 ml/100 lb (0.5 mg/kg) of body weight. At processing, blood was collected from the coccygeal vein from a convenience sampled population of calves from each load. Convenience sampling refers to the sampling of calves based on the availability of the investigator for collection of blood samples. Blood samples were placed in lithium heparin tubes and immediately placed on ice until transport to the laboratory. Troponin I assays were performed within 12 hours on all samples after calf processing had been completed.
After processing, all calves were monitored twice daily by trained personnel who were blinded to all cTn-I results. All calves showing clinical signs of BRDC (increased respiratory effort, nasal discharge, depression as evidenced by failure to rise and eat, low head and neck posture, or lethargy, rectal temperature > 40 oC) were treated in accordance with the pre-determined standard protocol (First BRDC treatment: enrofloxacin, second BRDC treatment: florfenicol, third BRDC treatment: oxytetracycline). All antimicrobials were administered according to label instructions. Calves that were treated for BRDC were selected for cTn-I sampling on days investigators were present for treatments and could confirm inclusion criterion. For enrolled calves, a blood sample was obtained from the coccygeal vein prior to administration of antimicrobials. Samples were placed in lithium heparin tubes on ice, and maintained in refrigeration until analyzed later that day.
Necropsy examination was performed on all calves that succumbed to disease or were euthanatized because of progression of BRDC during the course of the study. Necropsy findings of this subset of calves were used as an internal audit for accuracy of clinical case definition.
All hypothesis testing was performed with P-values of < 0.05 considered significant. Serum samples from 4 calves were used to perform quality control on the unit, handling, and testing procedures. The coefficient of variation was calculated and repeatability examined against the expected standards for the analyzer unit. Statistical analysis for the study data where all animals were independent and sampled a single time was done using a Student’s T-test to compare mean cTn-I values between clinically normal and diseased animals. Generalized linear models were used to evaluate potential associations among cTnl value with time and BRD status for calves enrolled Study II. The arrival lot and pen were included in the models as random effects to account for lack of independence between test subjects, and calf identification was used as a random variable to account for repeated measures on animals. Associations between serum concentration of cTnI and subsequent health outcomes of re-treatment and death were also evaluated using models specified in the same manner.
Results of repeated measurement of serum concentration of bovine cTnI yielded small CV’s and small SD (see Table 1). This was followed by measurement of serum concentration of cTnI in 16 calves of which 7 were normal calves and 9 calves had clinical and necropsy signs of BRDC but no evidence of myocardial injury. Calves in the BRD positive group exhibited gross pulmonary and pleural lesions associated with BRD which were in various stages of chronicity including pulmonary consolidation in several calves. Lesions involving the heart were not evident. Troponin-I was significantly (P = 0.036) greater in calves having respiratory disease (0.04 +/- 0.04 ng/ml) when compared with calves not having respiratory disease (0.01 +/- 0.01 ng/ml) (Figure 1).
The stocker study included a total of 293 calves that arrived in the three loads over a three day period with a mean arrival weight of 220 kg (485 lbs). Blood was collected for cTn-I analysis at arrival from 35 calves without clinical signs of BRDC selected from each of the three loads (n = 14, 11, and 10). During the 43 day back-grounding phase, 50.8% of the total population was treated for BRDC and 9.6% of the calves died. Necropsies were performed on all animals that died. A total of 28 calves died; 27 calves suffered severe bronchopneumonia and 1 calf died from causes unrelated to BRDC. This calf was excluded from cTn I data analysis.
A total of 66 calves meeting the treatment criterion for BRDC were sampled on 12 occasions during the first 31 days on feed. Of these 66 calves, only 6 had been randomly sampled at arrival to the facility. Samples of treated calves were acquired either at first (n = 47), second (n = 9), or third (n = 10) treatment for BRD. Blood concentration of cTn-I in calves at arrival (0.01 +/- 0.02 ng/ml) and in calves that remained healthy throughout the study period (0.01 +/- 0.02 ng/ml) were significantly less when compared with calves that became ill from BRDC at any time point (0.05 +/- 0.01 ng/ml) (P = 0.04; Figure 2). After accounting for treatment status of calves that developed BRDC, LSM cTn-l values did not differ between samples taken at any of the 3 treatment points (first, second, or third).
When Troponin-I concentrations were examined for samples taken at first treatment for BRDC, no differences in LSM cTn-I were identified based on subsequent retreatment status. However, inclusion of calf death as a factor illustrated an interaction (p = 0.055) between the timing of the sample and subsequent calf death. Calves that developed BRDC and survived had a serum cTn I concentration at first pull of 0.03 +/- 0.05 ng/ml. Calves that developed BRDC and died had a serum cTn I concentration of 0.10 +/- 0.14 ng/ml. cTn-I concentrations were significantly greater for calves that subsequently died when measured on first or third treatment when compared to cohort calves measured at this same time period (P < 0.05; Figure 3). Sample size limited our ability to examine the temporal relationship between blood concentration of cTn I and occurrence of death. However, scatter plot analysis revealed that calves with the largest cTn I died more quickly after treatment than did calves having lower cTn I.
Assessment of serum concentrations of cTnI may be useful in patients suffering non-cardiac diseases. Troponin assays may have variable accuracy and precision across species. Human cTn T assays have been shown to be unreliable for detection of bovine cTn T [27]. However, the homology of bovine and human cTn I has been reported be > 96% [28]. A commercial immunoassay has been validated for measurement of bovine cTnI [29]. In that study, the authors found that intra-assay precision was 4.48% and inter-assay precision was 13.36%. The authors concluded that this immunoassay, designed for detection of human cTn I was valid for use in cattle. Although cTn I is widely used as a screening test for myocardial infacrtion, increases in serum cTn I has been observed in human patients having a wide variety of cardiogenic (e.g. congestive heart failure, myocarditis, myocardial contusion, atrial fibrillation) and non-cardiogenic (e.g. sepsis, hypovolemia, pulmonary embolism, renal failure) diseases [29]. Despite this wide range of clinical syndromes, cTn I has prognostic value in these patients. Assessment of serum concentrations of Troponin I may be useful in patients suffering non-cardiac diseases. Although cTn I is widely used as a screening test for myocardial infarction, increases in serum cTn I have been observed in human patients having a wide variety of illnesses including cardiogenic (e.g. congestive heart failure, myocarditis, myocardial contusion, atrial fibrillation) and non-cardiogenic (e.g. sepsis, hypovolemia, pulmonary embolism, renal failure) diseases. Despite this wide range of clinical syndromes, cTn I has important prognostic value in these patients. Blood concentration of cTn I was found to be useful in assessment of risk of morbidity and mortality in human patients suffering acute pulmonary embolism [30]. Patients having increased serum concentrations of cTn I were more likely to suffer complications, recurrence of pulmonary embolization, or death. As an example, cTn I has been shown to have utility in assessment of the severity of acute pulmonary embolism in human patients [30]. In that study, patients having increased serum concentrations of cTn I were more likely to suffer complications, recurrence of pulmonary embolization, or death.
These findings suggest that Troponin I may be useful in determining severity of disease in cattle suffering pulmonary disease. Several recently published studies have reported blood cTn I concentration from cattle having cardiac and non-cardiac intra-thoracic diseases [23-27,31]. These reports included pericarditis, endocarditis, congenital heart disease, monensin toxicity, endotoxemia, reticulitis, mediastinal abscess, thymic lymphoma, caudal vena cava thrombosis, and chronic suppurative pneumonia. In a study examining cTn I in 34 normal cattle and 5 cattle having pericarditis, cTn I was markedly increased in 4 of 5 cattle effected with pericarditis [27]. Peek suggested a reference range of cTn I for cattle of < 0.08 ng/ml [26]. This is greater than that suggested by our study in which normal feedlot aged cattle consistently had cTnI of < 0.03 ng/ml. Using this cut-off point, troponin I was increased in 79% of cattle having primary bacterial cardiac disease. In that study, 40% of cattle having thoracic but non-cardiac disease had increased cTn I. However, the authors concluded that cTnI could not differentiate primary cardiac diseases from intrathoracic but non-cardiac disorders. In a study of 20 cattle having traumatic reticuloperitonitis, cTn I was considered normal when < 0.05 ng/ml and cattle having myocardial injury had cTn I of 3.26 +/- 2.1 ng/ml [27]. This cut off point is more consistent with the findings of our study. In these various studies, the authors’ noted that cTn I should be further investigated to determine its prognostic value because their study was limited to cattle having post-mortem examination done.
The reported extent of lung involvement in calves necropsied after having died with BRDC varies from < 33% of the lung volume in 30% of cattle, 33 to 66% in 58% of calves, and > 66% in 8.8% of calves [32]. Prolonged respiratory acidosis, hypercapnea, hypoxemia, and pulmonary hypertension may play a role in secondary myocardial injury leading to increasing concentrations of cTn I in the blood [33]. Although BRDC is often isolated to the lung tissues, primary and secondary myocardial injury occurs in some calves [11,34-36]. In a review of necropsy findings from 237 deaths associated with BRDC, findings other than pneumonia included pleuritis in 49% of effected calves, septicemia in 0.9%, and emboli in 0.5% [34]. Interestingly, we showed that cTn I was not statistically different from control calves at the time of the second treatment for BRDC. The significant increase in serum cTn I at the time of first and third treatments may be indicative of the severity and extent of the disease. Lesion severity has been associated with outcome in previous studies in feedlot calves [34]. Significant differences in cTnI between surviving and non-surviving calves treated at the first episode of BRDC may reflect the severity of the pathogen and rapid progression of pneumonia. The lack of difference in cTnI serum concentration among second treatment surviving and non-surviving calves is assumed to have been a result of the stage of progression of the pneumonia. Significant differences in cTnI between surviving and non-surviving calves treated at the third episode of BRDC may reflect chronicity of pneumonia resulting in consolidation, pulmonary fibrosis, and resulting hypertension. The concentration of Troponin I found in blood is expected to be proportional to the damage inflicted. Myocardial injury can be caused by many different insults including ischemia due to myocardial infarction, toxicity caused by ingestion of ionophores, nutritional deficiencies such as selenium deficiency, trauma caused by vehicular accidents, hypoxemia caused by anemia, severe pneumonia, or pulmonary interstitial disease, and increased cardiac work such as occurs with pulmonary hypertension [23-27,32-36]. For the purposes of this study, we hypothesized that many calves that fail to respond to antimicrobial therapy are calves in which BRDC has caused permanent pulmonary debilitation. We speculated that some calves failing to respond to BRDC treatment in feedlot settings are in fact suffering effects of primary, and perhaps permanent, pulmonary tissue injury rather than failure of response to antimicrobials. For calves that lived, the findings in the high-risk study indicated that cTnI concentrations were not elevated at initial treatment compared to that of arrival. Thus, low cTn I was suggestive of BRDC severity expected to respond to therapy or for spontaneous recovery to occur. When the ultimate outcome was death, cTnI levels at the first or third treatment were significantly greater than the baseline established upon arrival (Figure 3). Thus, marked increases in cTn I suggested more severe disease unlikely to respond to therapy or for recovery to occur. Accurate knowledge of animal prognosis based on a test done prior to treatment could result in greater efficiency of health care and reduced calf suffering because of early identification of calves that will become chronic poor performers. An economic benefit may also be realized by the producer and potentially limit the unnecessary use of antimicrobial drugs. In this scenario, cost savings are associated with reduced drug input, reduced feed use, reduced labor use, and improved facilities use.
The similarity in cTnI among groups at arrival suggests that pre-existing BRDC was not prevalent in this group of stocker calves. Some of these calves developed BRDC and subsequently died and this clinical course of disease was concomitant with a significant increase in blood cTnI concentration. These data illustrate that measurement of cTnI may have potential as a prognostic metric for clinical cases of BRD, but appears to be dependent on when the sample is taken relative to disease progression. Further research is warranted regarding the field applications of cTn I in stocker cattle. This test can be performed in field settings with rapid tests results. This makes troponin an attractive alternative to traditional treatment response tests. Further research will be necessary to elucidate the parameters for selection of calves for testing and to develop precise test interpretation cut-points.
The results of this study suggest that measurement of cTnImay be useful in the discrimination of calves having BRDC and, specifically, those calves at greatest risk of death. Study I demonstrated acceptable repeatability of the hand held device for measurement of bovine serum concentration of cTnI and revealed differences in mean blood cTnI concentration between clinically normal and chronic BRDC calves. These findings were supported by similar findings noted in the high-risk calf trial when comparing samples taken at arrival to blood cTnI concentration at first treatment for BRDC. In study I, calves without clinical signs of disease showed very little variability in cTn I measurements compared to the BRDC calves. This finding may be due to either the stage of disease progression, or individual variability in response subjects. Serum concentration of cTnI is close to the lower detection limit of the hand held device. More research should be performed to evaluate the utility of this test for both diagnostic and prognostic value related to respiratory disease in cattle.
The authors would like to acknowledge the technical support of Dr. Thomas Overbay and the Abaxis provided the cTnI test cartridges.
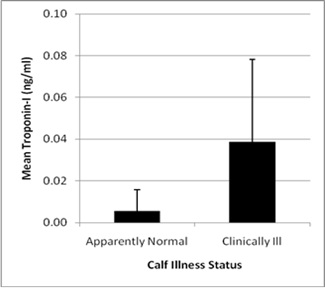 |
Figure 1: Mean blood concentration of cardiac Troponin I (cTn-I, ng/ml) in mixed breed beef calves (n -16; healthy n = 7; Pneumonia calves n = 9) presented with or without clinical signs of respiratory disease (P = 0.036) |
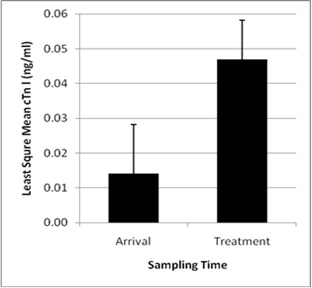 |
Figure 2: Least square mean blood concentration of cardiac Troponin I (cTn-I, ng/ml) values in stocker calves (total study population, n = 95) at arrival (n = 35) and at treatment for respiratory disease (n = 66, 6 of which were previously sampled as normal at arrival) at any time point during the 42 days on feed after arrival (P = 0.04) |
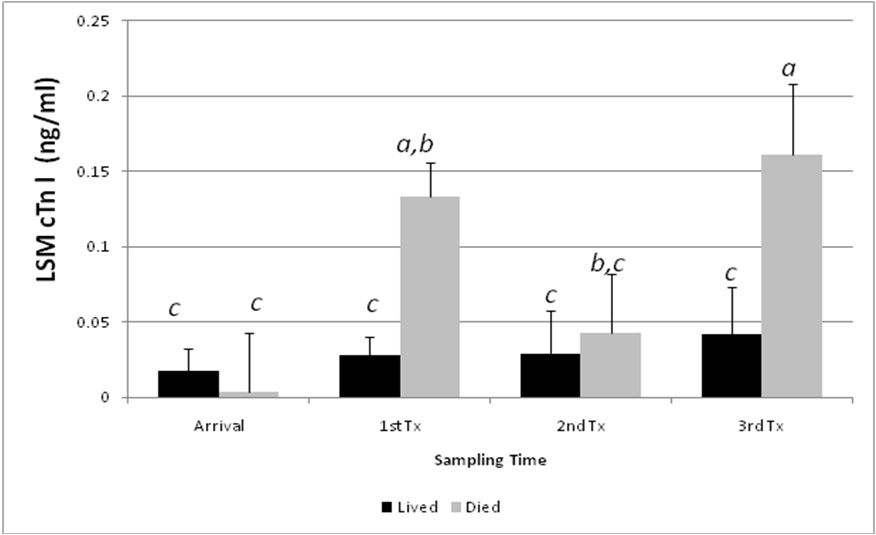 |
Figure 3: Interactive least square mean blood concentration of cardiac Troponin I (cTn-I, ng/ml) in stocker calves by sampling time period and subsequent health outcome (death). Data represent analysis of serum samples collected at arrival (n = 35); first BRDC treatment (n = 47); second BRDC treatment (n = 9); and third BRDC treatment (n = 10). Columns that differ by superscript are significantly different (p < 0.05) |
Calf ID |
cTnI-1 ng/ml |
cTnI-2 ng/ml |
cTn-3 ng/ml |
Mean ng/ml |
SD ng/ml |
CV (%) |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0.01 |
0 |
0 |
0.003 |
0.006 |
17.3 |
3 |
0.02 |
0.02 |
0.02 |
0.02 |
0 |
0 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
| Table 1 : Assessment of repeatability of cTnI measurement in healthy calves using a iSTAT hand-held analyzer; Each analysis was performed in triplicate and mean, SD< and CV determined | ||||||






































