Top Links
Journal of Veterinary Science and Animal Husbandry
ISSN: 2348-9790
Safety of Permethrin and Pyriproxyfen in Dogs Treated With VetGuard Plus®
Copyright: © 2016 Vega NM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Ectoparasites are a continuous problem in small companion animals, and veterinary personnel and pet owners are constantly exposed to transferable residues when handling dogs treated with topical ectoparasiticides. This study aimed to measure the transferable residues of permethrin and pyriproxyfen in the blood and hair coat of six dogs treated topically with VetGuard Plus®. Between 4-5 mL of blood were collected in EDTA tubes via jugular venipuncture at 0, 1, 2, and 3 days after VetGuard Plus® application. Glove samples for pesticides residue measurement were collected at days 0, 1, 2, 3, 4, 7, 14, 21, 28, and 35, during which physical parameters such as heart and respiration rate, body weight, and body temperature were also measured. Gloves were collected by petting each dog’s hair coat for 5 minutes using new 100% cotton gloves each time. Permethrin and pyriproxyfen residues were extracted with methylene chloride: petroleum ether (1:1) and analyzed using gas chromatograph/mass spectrometer (GC/MS). Identification and confirmation of permethrin and pyriproxyfen were made based on characteristic ions, and quantitation was done based on peak area. No residues were detected in the blood at any given interval. In the glove samples, the highest concentrations of permethrin and pyriproxyfen were detected at 24 h (417.52 ± 58.38 ppm, and 30.51 ± 8.52 ppm, respectively). The lowest concentrations of permethrin and pyriproxyfen were detected at 35 days (2.49 ± 0.43 ppm, and 0.04 ± 0.03 ppm, respectively). None of the dogs showed any adverse physical or behavioral reactions. In conclusion, VetGuard Plus® is a safe product for dogs and poses no significant risk to veterinary personnel and pet owners if proper precautions are taken.
Keywords: VetGuard Plus®; Dogs; Permethrin; Pyriproxyfen; Ectoparasiticide safety; Pyrethroids; Insect growth regulators
Ectoparasites are a constant problem in dogs, and their potential to transmit zoonotic pathogens makes them a major public health concern worldwide. Cat fleas (Ctenocephalides felis) are the most important ectoparasite of dogs and cats worldwide. They can transmit typhus, spotted fever, and cat-scratch disease. They also serve as hosts for the tapeworm (Dipylidium caninum) [1-3]. Lyme disease is transmitted by Ixodes scapularis, a tick that also acts as a vector for Babesis microti (human Babesiosis) and Ehrlichia phagocytophila (human granulocytic ehrlichiosis) [4]. Chewing lice (Trichodectes canis) are also vectors for tapeworm (Dipylidium caninum), and can cause itching, scratching, hair loss, and anemia. Finally, the mosquito (Anopheles quadrimaculatus) is the primary vector of malaria in the United States, and it can also transmit heartworms and the West Nile virus [5].
VetGuard Plus® (TruProdigy, Eagle, ID, USA) is an over-the-counter topical flea and tick treatment that contains 45.0% of the pyrethroid insecticide permethrin (3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester)) and 5.0% of the insecticide growth regulator (IGR) pyriproxyfen (4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether); Figure 1 illustrates the chemical structures of both compounds (ChemSketch Software). This product has three primary functions: 1) to kill fleas (Ctenocephalides felis), ticks (Ixodes scapularis), chewing lice (Trichodectes canis), and mosquitoes (Anopheles quadrimaculatus) for up to one month; 2) to repel fleas, ticks, and mosquitoes for up to one month; and 3) to prevent flea eggs from maturing into adults for up to 4 months. VetGuard Plus® should only be used in dogs that weigh at least 5 pounds (2.25 kg) and are 12 weeks of age or older. Once topically applied, the active ingredients are absorbed and distributed primarily throughout the sebaceous glands of the skin, which continuously release the active ingredient residues into the skin and hair coat [6-8].
Permethrin is a synthetic pyrethroid used as a topical insecticide. It was invented in 1973 and first registered in the United States for use on cotton in 1979, and is the most commonly used pyrethroid to this day. Pyrethroids are synthetic pyrethrins, which are derived from the flowers of Chrysanthemum cinerariaefolium, and can be classified as type I and type II based on their chemistry and the type of syndromes they produce. The broad spectrum activity of permethrin allows it to kill ticks, fleas, lice, mosquitoes, flies, termites, and cockroaches [9].
Pyriproxyfen is a broad spectrum IGR and pyridine-based pesticide that was first used in the United States in cotton pest resistance management programs [10]. Pyridines are one of the main ingredients found in pesticides, and they are derived from the roots and leaves of Althaea officinalis (Marsh mallow) and Atropa belladonna (Belladonna). Pyriproxyfen affects the embryonic, larval, and reproductive stages of fleas, whiteflies, houseflies, boll weevils, cockroaches, and mosquitoes [11,12].
Currently, there are no studies that indicate the safety of VetGuard Plus® in dogs, nor the levels of exposure humans may have to the active ingredients of this product. Veterinary personnel and pet owners are constantly exposed to transferable residues when handling dogs treated with topical ectoparasiticides, which can be a health and occupational hazard. Therefore, this study aimed to assess the safety of VetGuard Plus® in dogs and the potential of residue transfer to humans.
A total of six healthy adult dogs (mixed breed, between 45-60 pounds, medium hair coat) were used in this study, and were all part of one experimental group. The dogs were volunteered by Murray State University students and staff, who all signed a consent form of participation. No ectoparasiticides or heartworm preventives were applied to the dogs for at least one month prior to the start date, and they did not come in contact with water for at least three days before and during this study to ensure the proper absorption of VetGuard Plus®. Blood and glove samples were taken prior to this investigation, and tested negative for permethrin and pyriproxyfen residues.
VetGuard Plus® for large dogs (33-66 pounds) was purchased from Walmart (Clarksville, IN). The product came in individual tubes designed for a single application, each one containing 3.0 mL of the product solution (45.0% permethrin and 5.0% pyriproxyfen). Technical grade permethrin (99.5%) and pyriproxyfen (98.9%) were purchased from Chem Service, Inc. (West Chester, PA). All other chemicals of highest purity were obtained from Fisher Scientific (Fair Lawn, NJ).
The experimental design and number of animals in this study was based upon previous studies conducted at Murray State University and Breathitt Veterinary Center [7,8,13]. An application for the use of the animals was submitted to and approved by Murray State University’s Institutional Animal Care and Use Committee (IACUC). The samples were collected at Murray State University’s Department of Animal Health Technology, and the sample extraction and analysis were carried out at Breathitt Veterinary Center’s Department of Toxicology.
A single tube of VetGuard Plus® (3.0 mL) was evenly applied to each dog in three different spots according to the manufacturer’s instructions: between the shoulder blades, in the middle of the back, and at the base of the tail.
Physical parameters such as body weight, heart rate, respiration rate, and rectal temperature were measured at the time of VetGuard Plus® application and during each sample collection. Skin condition, presence of fleas and/or ticks, and adverse side effects were also evaluated at the sites of product application during each interval. Owners were asked whether they had noticed any changes in their dog’s skin condition and behavior throughout all evaluations.
Blood and glove sampling was performed in each dog to measure the amount of transferable permethrin and pyriproxyfen. Blood samples were collected via jugular venipuncture at 24, 48, and 72 hours, and 1-week post-application of VetGuard Plus®, using a 5 mL syringe with a 22-gauge needle. Approximately 4 mL of blood was collected in EDTA tubes, and the samples were refrigerated at 2 °C until analyzed (<24-48 hours). Glove samples were collected using the wipe sampling technique, which consisted of petting the dog’s hair coat in a back-and-forth motion while wearing one 100% cotton glove. The petting was done along the back and sides of the hair coat, while avoiding the application site, for a period of 5 minutes at each time interval. After sampling, each glove was immediately stored in a clean 473 ml (one pint) glass jar at room temperature until analyzed (<24-48 hours).
Each blood sample was transferred from the EDTA tube into a 125 mL separatory funnel and weighed. Next, 25 mL of methylene chloride: petroleum ether (1:1) was added to each funnel, after which it was shaken three times and allowed to vent in between each shaking. After 15 minutes, the solvent was drawn off from the top with a disposable pipette and dispensed through a sodium sulfate filled filter into a 100 mL beaker. The solvent was allowed to evaporate overnight, and was reconstituted the next morning in methylene chloride: petroleum ether (1:1) before undergoing GC/MS analysis [7,8,13].
Each glove sample was removed from the glass jar and weighed, after which they were placed into individual 250 mL beakers. Next, 100 mL of methylene chloride: petroleum ether (1:1) was added to each beaker until the gloves were completely covered. After the gloves were submerged for 30 minutes, the solvent was poured through a sodium sulfate filled filter into a 100 mL beaker and allowed to evaporate overnight. The next morning, the residue was reconstituted in methylene chloride: petroleum ether (1:1) before undergoing GC/MS analysis [7,8,13].
The permethrin and pyriproxyfen residues extracted from the blood and glove samples were analyzed and quantified using an Agilent Gas Chromatograph (GC model 7890A)/Mass Spectrometer (MS model 5975C) coupled with a computer. The residues were measured in terms of μg/g and expressed as ppm, and graphs were developed to compare the residue levels measured at each time interval (Mean ± SEM). Statistical analysis was performed using the NCSS9 Statistical Analysis and Graphics software for Windows®.
After evaporating overnight, the samples were reconstituted with methylene chloride: petroleum ether in an appropriate volume, after which they were passed through a Sep-Pak® cartridge (Waters Corp, Milford, MA). One μL of the sample extract was injected via the injector of the GC. A capillary column (25 m x 0.52 μm Ultra II Cross) was used, which had a 5% phenyl methyl siloxane coating and it was directly connected to the Mass Selective Detector via an interface and heated transfer line. Ultrapure (99.9999%) helium was used as the carrier gas at a flow rate of 2.3 mL/min. The injector’s temperature was 200 oC and it was operated in the splitless mode. A temperature program was used for the chromatograph’s oven, starting with a temperature of 100 oC and increasing in 20 oC/min increments until reaching a final temperature of 300 oC, which was maintained for 5 minutes. Each injection run had a duration of 16 minutes, with a solvent delay of 5 minutes. The transfer line temperature was 280 oC, and the source temperature was 230 oC. The instrument was operated in electron ionization mode, and the ion energy was 70eV.
Two peaks of permethrin were eluted at 10.047 min (cis isomer) and 10.110 min (trans isomer), respectively, while the single peak of pyriproxyfen was eluted at 9.553 min. The GC/MS had a sensitivity for these compounds in the ng range, and the limit of detection was in the low μg/g (ppm) range. The total ion chromatogram for permethrin can be seen in Figure 2, while Figure 3 shows a mass spectrum with characteristic ions for permethrin (93.1, 127.0, 163.0, 183.1, 255, and 390.0). Figure 4 demonstrates the total ion chromatogram for pyriproxyfen, and the mass spectrum with characteristic ions for pyriproxyfen (51.1, 63.1, 78.1, 96.1, 136.1, 226.1, and 321.1) is shown in Figure 5.
Table 1 illustrates some of the physical parameters measured before and after the application of VetGuard Plus®. During some evaluations, it was not possible to evaluate respiration rate in some dogs due to panting; therefore, no statistical analysis was performed for this parameter. None of the dogs showed any significant differences in any of the parameters before and after application.
The label on VetGuard Plus® states that it can kill fleas, ticks, chewing lice, and mosquitoes for up to one month; repel fleas, ticks, and mosquitoes for up to one month; and prevent flea eggs from maturing into adults for up to 4 months [14]. Therefore, it was expected in this study that the concentration of the product’s residues (permethrin and pyriproxyfen) in the glove samples would be the highest at 24 hours post-application, and that the residues would be present in the hair coat for up to 30 days post-application.
No residue levels of either permethrin or pyriproxyfen were detected in the blood samples at any given time during this study. Figures 6 and 7 illustrate the permethrin and pyriproxyfen concentrations in the glove samples, respectively. The highest concentration of permethrin and pyriproxyfen was detected at 24 hours post-application (417.52 ± 58.38 ppm, and 30.51 ± 8.52 ppm, respectively). At 48 hours post-application, residues for both permethrin and pyriproxyfen showed decline (292.76 ± 38.41 and 20.06 ± 3.27 ppm, respectively). These concentrations continued to gradually decrease to insignificant levels over the course of the study. At 5 weeks, the level of permethrin was 2.49 ± 0.43 ppm, and for pyriproxyfen it was 0.04 ± 0.03 ppm.
No adverse physical or behavioral side effects were observed throughout the duration of the study.
The purpose of this study was to determine the levels of permethrin and pyriproxyfen in the bloodstream and on the hair coat of dogs during a 5-week period following a single topical application of VetGuard Plus®. In turn, this allowed assessment of the safety and toxicity of the product in dogs. It also helped simulate the degree of exposure of transferable residues that veterinary personnel and dog owners would receive from dogs treated with VetGuard Plus®.
Based on the toxicity data of permethrin (oral LD50 in rats 430-4000 mg/kg and in mice 540-2690 mg/kg; and dermal LD50 in rabbits >2000 mg/kg) and of pyriproxyfen (oral and dermal LD50 in rats >5000 mg/kg and >2000 mg/kg, respectively), findings of the present investigation indicated a high level of safety of VetGuard Plus® as a topical ectoparasiticide. Both permethrin and pyriproxyfen residues were detected in the hair coat of all the candidates, and their highest concentrations were measured at 24 hours (417.52 ± 58.38 ppm, and 30.51 ± 8.52 ppm, respectively) post-application of the product. This coincides with the product’s label, which states that it may take from 24 to 48 hours for the product to be absorbed throughout the dog’s skin. This is also indicative that humans and other animals, especially cats, have the highest risk of exposure during this time. No permethrin or pyriproxyfen residues were detected in the blood at any given time of the study, which suggests a high level of safety for veterinary personnel when coming in contact with blood of VetGuard Plus®-treated dogs during emergency or surgical situations. In similar studies with ectoparasiticides such as BioSpot Defense®, Activyl®, RevolutionTM, Advantage®, Certifect®, and FrontlineTM, transferable residues of selamectin, imidacloprid, permethrin, etofenprox, and amitraz were found in the dog blood, and residues of etofenprox, permethrin, idoxacarb, selamectin, imidacloprid, amitraz, and fipronil were found in significantly greater amounts on the hair coat of treated dogs [6,7,13,15-17].
Pyrethroids, such as permethrin, exert neurotoxicity by targeting primarily the voltage-gated sodium channels of nerve cell membranes [18,19]. By binding to specific sodium channel proteins, pyrethroids slow down the closing and opening of these channels, which causes the nervous system to become hyper-excited due to the increased amount of sodium entering the channels [18,20,21]. Gamma aminobutyric acid (GABA)-gated chloride channels and calcium channels have been implicated as additional sites of action [22]. The classification of pyrethroids is based on chemistry and two different syndromes: T (tremors) and CS (choreoathetosis/ salivation). The T syndrome is mostly caused by Type I pyrethroids (no alpha-cyano group), which affect the central and peripheral nervous systems, while the CS syndrome is caused by Type II pyrethroids (contain alpha-cyano group), which mostly affect the central nervous system. The main mechanism of action of Type I pyrethroids (such as permethrin) is primarily axonal sodium channel depolarization causing repetitive nerve impulses [22]. Type II pyrethroids work by depolarizing the cell membrane [18,19,22,23]. Evidence suggests that the mechanism of action differs in toxicity of type I and type II pyrethroids [22,24]. In humans, dermal exposure to permethrin can cause irritation, itching, and paresthesia, while ingestion may cause nausea, vomiting, sore throat, abdominal pain, coma, convulsions and death [25-27]. Studies have shown that dermal exposure of permethrin has caused irritation in rabbits, and ingestion of the product has caused transient signs in dogs, including tremors [18,28]. Permethrin is highly toxic to cats and the toxicity often occurs following exposure to a highly concentrated (45-65%) spot-on permethrin product designed for canines, either by direct application, grooming, or being in close contact with recently treated dogs [18,29]. Clinical signs may include tremors, muscle fasciculations, twitches, seizures, ataxia, mydriasis, temporary blindness, and death [30].
Pyriproxyfen interferes with the insect’s endocrine system and acts as an analog of the juvenile hormone (JH), mimicking its effects and preventing the molting process, which consequently halts further reproduction [31]. It can affect insects through dermal or oral exposure [11]. In cat fleas, which are the most important ectoparasites in dogs and cats alike, pyriproxyfen accumulates in the developing larvae and mimics the JH in order to maintain genes that aim production of larval cuticle. Consequently, this prevents fleas from completing the larval-pupal or pupal-adult molt during metamorphosis, turning them into non-functioning and non-reproductive intermediates [32]. Pyriproxyfen is relatively safe to use in mammals, and is classified by the EPA as not likely to cause cancer in humans [11]. According to the World Health Organization, the oral and dermal LD50 for pyriproxyfen in rats is over 5000 mg/kg and over 2000 mg/kg, respectively. Skin and eye irritation tests done with pyriproxyfen in rabbits showed non-irritating and minimally irritating results [12,33]. Although pyrethroids have a lower toxicity in mammals and are considered safer, adverse effects caused by pyridines usually arise after long-term exposure [34].
Although this study aimed to mimic the exposure of veterinary personnel to transferable residues in dogs treated with ectoparasiticides, it was limited to a controlled number of exposures for a period of only 5 weeks. Additionally, the dogs were petted with 100% cotton gloves, which tend to absorb more of a given substance as compared to bare skin [35]. In reality, veterinary personnel (especially those working in general practice) are typically exposed to several dogs on a daily basis, and personal protective equipment (PPE) such as gloves are not always worn by the staff while handling dogs. The study was also limited to dogs with a medium-length hair coat, and each dog’s hair coat had a different thickness. Also, there were several breeds of dogs, and their ages and weights ranged from 1-11 years and 45-60 lbs, respectively. Several anatomical factors such as weight, hair follicle density, skin thickness, age, and breed difference “can influence barrier function and thus the absorption of chemicals into the skin” [36]. These factors could have potentially influenced the rate of dermal residue absorption in the dogs, as well as the detected levels of residues in the gloves. For future studies with VetGuard Plus®, it would be beneficial to use candidates of similar age, hair coat thickness, and breed. Also, considering that VetGuard Plus® is designed for monthly applications, similar studies could be extended to longer periods of time in order to assess potential adverse side effects, presence in the blood, and vital organ function.
There are many factors that can influence the amount of transferable residue that people are exposed to when coming in contact with dogs topically treated with ectoparasiticides. People who work with and handle animals on a daily basis have a higher risk of exposure, including veterinary personnel, dog handlers and trainers, dog groomers, and even K9 officers. The presence of PPE such as gloves and masks, especially while applying the product and/or handling dogs treated within hours, can also affect the levels of dermal, inhalation, and oral exposure to transferable residues. Other factors include the number of daily exposures to topically treated dogs, proper application and compliance with the manufacturer’s instructions, and the age, breed, weight, hair coat length/density, skin thickness, and disease status of the animal [35,36].
A study conducted by Starr, et al., which measured the concentration of 13 pyrethroid pesticides and their metabolites in indoor dust samples of homes and daycares, found that permethrin was present in all samples [37]. The use of ectoparasiticides in dogs can certainly contribute to this; therefore, limiting the exposure to topical ectoparasiticides is crucial, and one of the most important steps in doing so is to apply the products following the specific manufacturer’s instructions. On occasions, even if ectoparasiticides are properly used and there is no resistance to the products, flea and tick infestations may take time to overcome depending on the weather, vegetation, wildlife host abundance, agricultural practices, etc., which may cause owners to overuse topical ectoparasiticides [38,39]. In turn, this can lead to higher levels of transferable residues on the dog’s hair coat and throughout the dog’s environment.
Like many other topical ectoparasiticides, VetGuard Plus® should be used in accordance with the dog’s weight and age. The product is sold by weight groups (small dogs between 5-15 lbs; medium dogs between 16-33 lbs; large dogs between 33-66 lbs; and extra-large dogs over 66 lbs), and the label states that it should be used in dogs that weigh at least 5 lbs and are 12 weeks of age or older. As reported by the EPA in 2009, the number of adverse events caused by spot-on flea and tick medications increased by 53% between 2007 and 2008, with young and small-breed dogs among the most affected [40]. Upon further investigation, it was theorized that this could have been caused by the products’ weight ranges being too wide, owners misusing the products, and/or small dogs being more prone to adverse reactions. By always purchasing the right dose and applying the product in compliance with the weight and age specifications, owners can limit their exposure to topical ectoparasiticide residues and decrease the occurrence of adverse effects on their pets. Owners should also be advised to wear gloves and other PPE while applying these products, as well as keeping their pet in an isolated area and avoiding direct contact with them for at least 24-48 hours following application. Veterinarians play a pivotal role when it comes to educating pet owners about ectoparasite infestations, as well as the proper use and safety precautions of topical ectoparasiticides.
The findings of this study suggest that VetGuard Plus® is a safe product for dogs and poses no significant risk to humans if proper precautions are taken. Following a single application of VetGuard Plus®, no adverse physical or behavioral side effects were observed throughout the duration of this study, suggesting a high level of safety in dogs. Topical permethrin and pyriproxyfen concentrations were the highest at 24 hours post-application of the product, representing a high risk period of exposure to humans. These concentrations continued to gradually decrease to insignificant levels over the 5-week period of the study. The absence of residues in the blood at all times suggests a high level of safety for veterinary personnel in emergency and surgical situations. Owners should always adhere to the manufacturer’s instructions when using topical ectoparasiticides to minimize exposure to transferable residues.
Studies with a larger sample size, as well as candidates with a similar weight, age, breed, and hair coat, should be performed to get a more accurate representation of the product’s safety. Also, considering that VetGuard Plus® is designed for monthly applications, similar studies could be extended to longer periods of time in order to assess potential adverse side effects, presence in the blood, and organ function. Future research should focus on determining the levels of permethrin and/or its metabolites (cis- and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid; and 3-phenoxybenzoic acid) and pyriproxyfen in canine urine, since this is another common route of residue exposure in veterinary clinics and households [23]. To expand more on the findings of this experiment, liver and kidney values should also be measured in dogs topically treated with VetGuard Plus® in order to assess the effects that permethrin and pyriproxyfen have on these vital organs.
Vega NM, major contributor; Case KM, research assistant; Gupta RC, thesis advisor; Doss RB, sample processing and data analysis; and Canerdy TD, graduate advisor.
The author would like to sincerely thank everyone at the Breathitt Veterinary Center and Murray State University involved with this study and the preparation of this manuscript. Also, many thanks to the owners who contributed to this research by volunteering their dogs and valuable time.
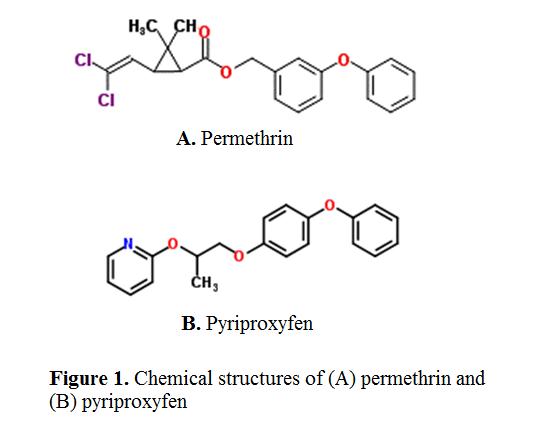 |
Figure 1: Chemical structures of (A) permethrin and (B) pyriproxyfen |
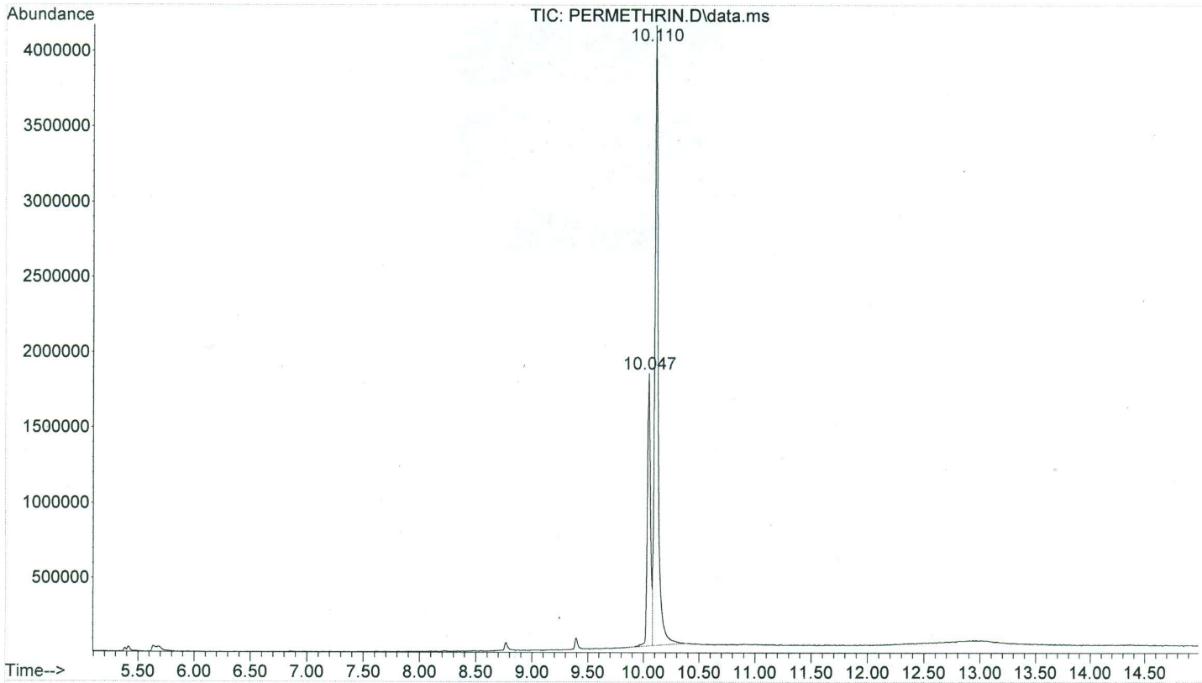 |
Figure 2: GC/MS-total ion chromatogram of permethrin |
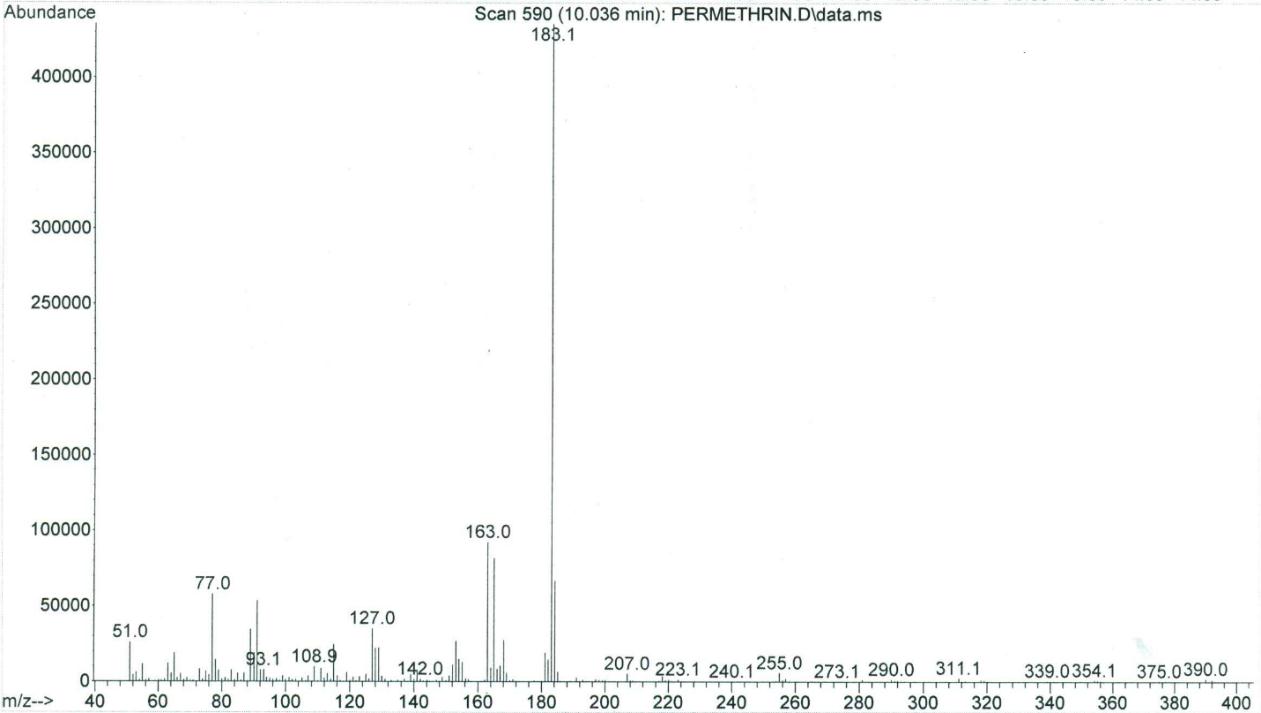 |
Figure 3: Mass spectrum of permethrin |
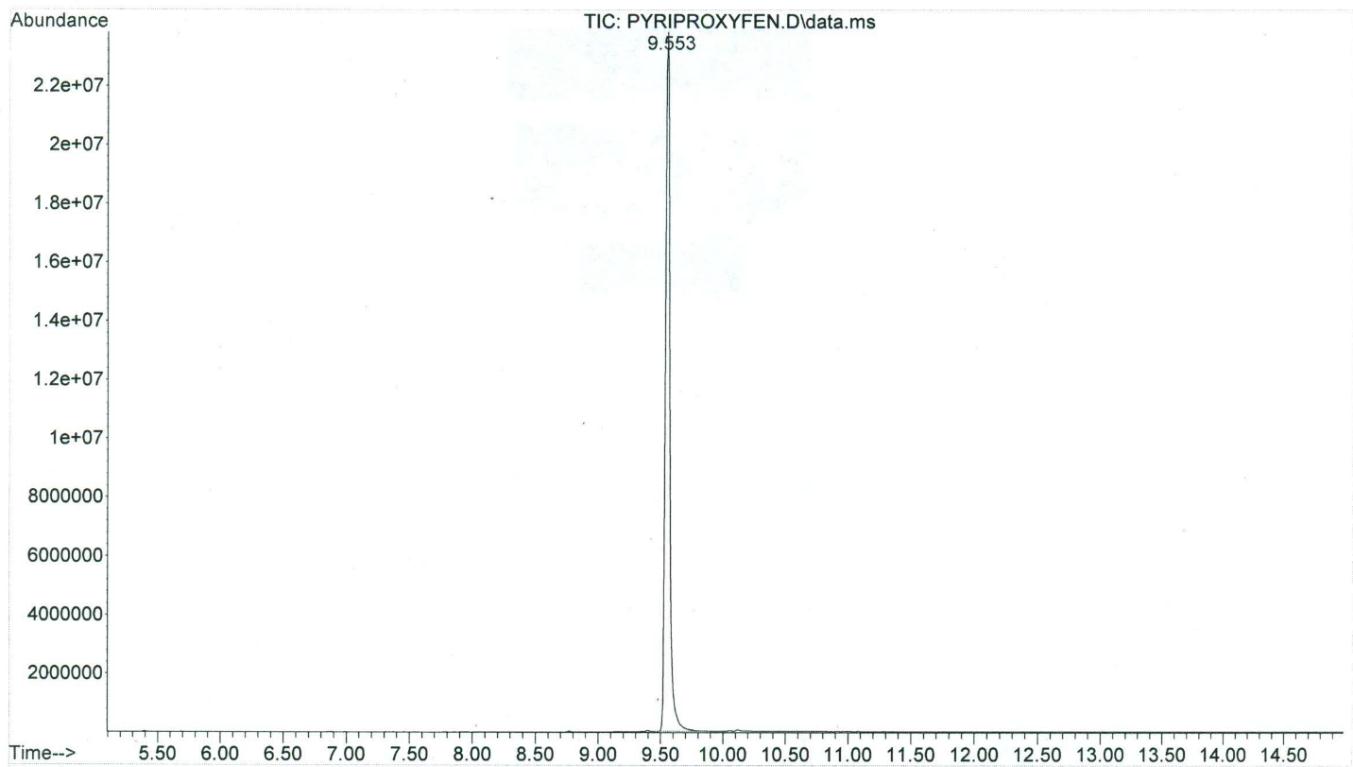 |
Figure 4: GC/MS-total ion chromatogram of pyriproxyfen |
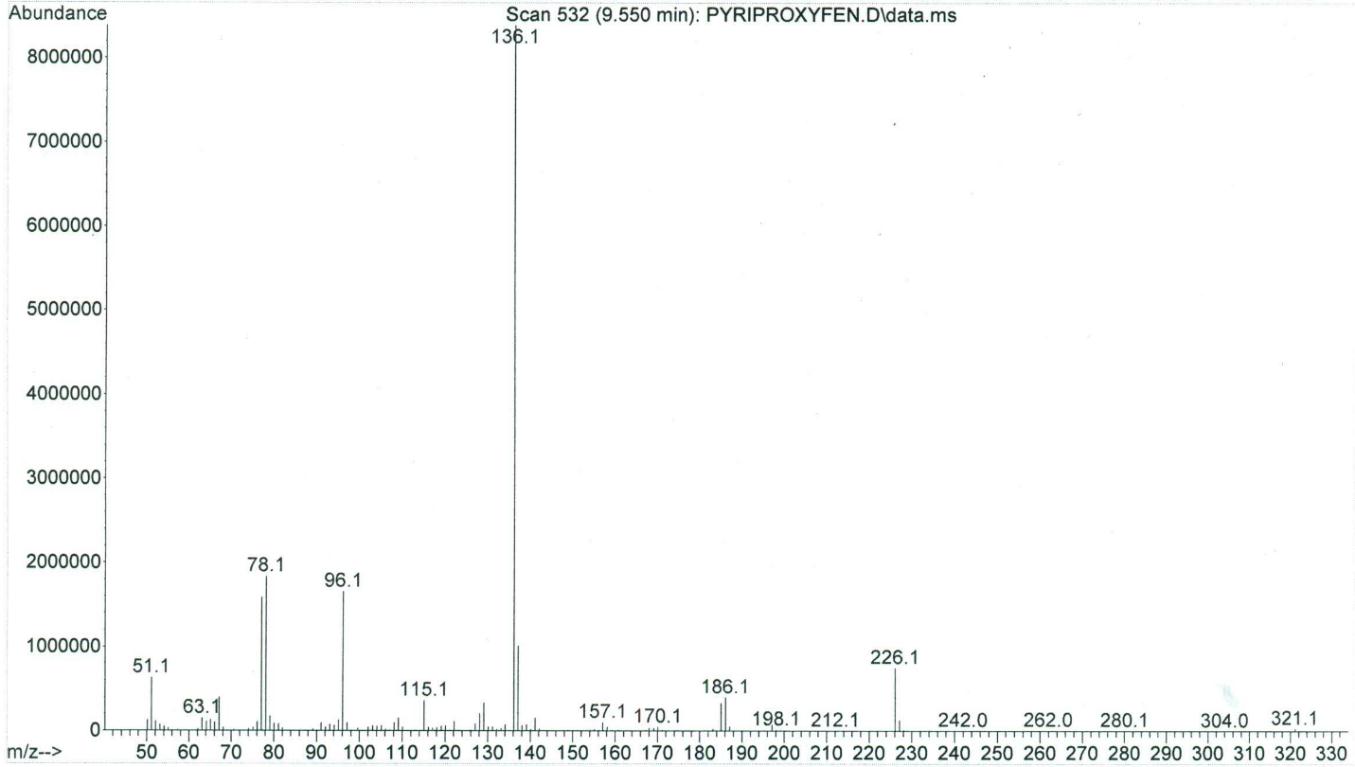 |
Figure 5: Mass spectrum of pyriproxyfen |
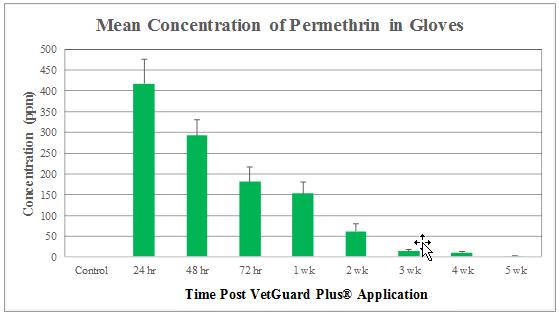 |
Figure 6: Concentrations of permethrin (µg/g glove or ppm) are presented as Mean ± SEM (n = 6) |
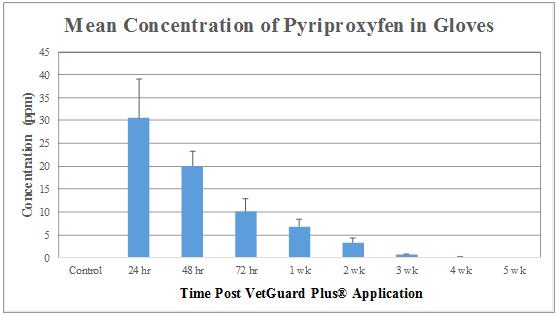 |
Figure 7: Concentrations of pyriproxyfen (µg/g glove or ppm) are presented as Mean ± SEM (n = 6) |
Post-application Time |
Body Weight (lbs) |
Heart Rate (bpm) |
Temperature (°F) |
Day 0 (control) |
53.23 ± 1.89 |
129.00 ± 9.98 |
101.21 ± 0.31 |
Day 1 (24 h) |
52.53 ± 2.14 |
140.00 ± 9.49 |
101.85 ± 0.38 |
Day 2 (48 h) |
52.40 ± 1.98 |
103.33 ± 7.19 |
101.35 ± 0.38 |
Day 3 (72 h) |
52.77 ± 2.05 |
105.83 ± 1.47 |
101.65 ± 0.34 |
Day 7 (1 week) |
52.37 ± 2.06 |
130.00 ± 8.56 |
101.67 ± 0.31 |
Day 14 (2 weeks) |
52.23 ± 1.91 |
122.67 ± 5.33 |
101.27 ± 0.44 |
Day 21 (3 weeks) |
53.28 ± 1.77 |
118.00 ± 7.50 |
101.10 ± 0.35 |
Day 28 (4 weeks) |
53.03 ± 2.28 |
116.67 ± 6.23 |
100.70 ± 0.46 |
Day 35 (5 weeks) |
53.17 ± 2.24 |
103.33 ± 3.33 |
100.63 ± 0.21 |
*No statistically significant difference from day 0 (P>0.05). Statistical analysis for respiration rate was not performed due to panting in some candidates |
|||






































