Top Links
Journal of Orthopaedics and Physiotherapy
ISSN: 2639-930X
Ultrasound as a New Imaging Tool to Assess Pathological Change of Joints in Preclinical Mouse Models of Osteoarthritis
Copyright: © 2018 Lin X. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Murine osteoarthritis (OA) models are important for exploring OA pathology and treatment in the pre-clinical study. Longitudinal in vivo imaging modalities, including X-ray, computed tomography and magnetic resonance imaging, are commonly used diagnostic tools in OA clinic, while end-point histomorphometry analysis is the major outcome measurement in pre-clinical study because clinical imaging modalities have limited resolution for small animals. Thus, developing new longitudinal in vivo imaging protocols for murine models of OA is a critical unmet need. Here, commonly used post-traumatic murine models of OA and the utilization of X-ray, computed tomography and magnetic resonance imaging techniques to monitor disease progression and treatment response in these models is reviewed. Then ultrasound (US) imaging, a widely used and cost-efficient tool, in arthritis clinic and its utilization in knee OA of patients is introduced. Finally, our experience of using US imaging in normal and OA mouse knees is described to demonstrate the feasibility of US as a new imaging tool to measure disease progression longitudinally.
Keywords: Osteoarthritis; Murine Models; Post-Traumatic; in vivo Imaging; Ultrasound
Osteoarthritis is the most common form of arthritis that affects millions of adults worldwide [1]. There are no effective drugs for OA and current treatments are mostly palliative. OA is a whole joint disease involving cartilage, subchondral bone and synovial soft tissue [2]. Many factors contribute to OA pathogenesis, including mechanical stress, biochemical abnormalities, and metabolic disorders [3]. Murine models are important for OA research for the ease of genetic manipulation and inflicting joint trauma by surgery [4]. A challenge for murine models of OA is the lack of non-invasive approaches that allow researchers to assess the severity and progression of OA diseases longitudinally. X-ray and magnetic resonance imaging (MRI) are widely used in OA patients with established quantitative and semi-quantitative assessment standards [5-8]. However, use of these imaging modalities in small animals, especially mice, is limited due to expensive and low resolution equipment, and operator dependence. Medical ultrasound (US) has also been used to evaluate knee OA disease activity in patients, demonstrating that US detected synovial inflammation and effusion is positively correlated with radiographic OA and clinical symptoms [9]. Our group applied US scan on joints of TNF-transgenic mice, a mouse model of rheumatoid arthritis (RA) and demonstrated that both joint space volume and power doppler (PD) volume can be used as outcome measures of joint inflammation and active synovitis [10,11]. We have started to use US scanning in mouse OA and obtained promising results [12]. Here, the advantages and limitations of existing imaging modalities in murine models of OA are reviewed. Our own experience using US imaging modality in mouse OA joints is described, and the possibility to use longitudinal US as a new approach to quantify joint soft tissue changes in a mouse model of OA is discussed.
Preclinical OA models have greatly improved our understanding of OA pathogenesis. Multiple animal species have been used, including canine, goat, pig, horse, and rhesus macaque [13-21]. Mouse OA models are the most commonly used preclinical OA models due to the ease of surgical or genetic manipulation, drug administration, and their relatively low cost. Murine models of OA are roughly categorized into the following groups: surgery-induced, genetic- and chemical-induced joint injury.
Surgery-induced OA: This OA model mimics a pathologic process of posttraumatic OA (PTOA) in humans. It is achieved via joint destabilization caused by various surgical procedures in the knees. A mouse knee joint is composed of hard tissues and surrounding soft tissues that we named synovial soft tissues. Hard tissues consist of articular cartilage of the femur (lateral or medial condyle) and tibia (tibia plateau) and subchondral bones. Soft tissues include ligaments (medial and lateral collateral, anterior and posterior cruciate, patellar, medial meniscus tibial/coronary line), the meniscus (medial and lateral), and the synovial membrane. By transecting or one or several joint ligaments in combination of removing a portion of medial meniscus, mice develop osteoarthritic changes at different paces, depending on the procedure (Figure 1). Among commonly used mouse PTOA models, the most severe cartilage damage is seen in knee that undergoes anterior cruciate ligament transaction (ACLT), followed by MLI (meniscal-ligamentous Injury)- transection of posterior cruciate ligament plus removal of medial meniscus and then by DMM (destabilization of the medial meniscus)-transection of the medial meniscus tibial ligament [22-25]. At the 2016 OARSI meeting, 133 out of over 900 abstracts used PTOA mouse models. Of these, 53 used the DMM model, 78 used the ACLT model and 2 used the MLI model. Surgical induced OA models ensure that OA is induced at the same time, thus minimizing the variation of the onset of OA in the genetic models. The combination of DMM and genetically modified animals are used to identify enzymes, proteins and transcriptional changes in OA pathogenesis [26-30]. In patients, PTOA is often diagnosed 15-20 years after the initial joint injury [31]. The DMM involves transecting the medial meniscal tibial ligament, which doesn’t exist in patients. The ACLT often causes acute joint damage and severe inflammation and the MLI requires highly experienced personnel. Despite these limitations, surgery-induced mouse models of OA are still most commonly used in pre-clinical studies.
Genetically modified mice with OA phenotypes: In different strains of wild-type mice, OA histological changes could occur spontaneously as mice aged, including C57Bl/6, BALB and Str/ort mice [32,36]. Although these spontaneously developed OA mice bear similar degenerative natures of human OA, it takes a long time to observe age-related OA changes. For instance, it takes 24-months for changes to become observable in C57Bl/6, 12-months in BALB mice, and 2.5-months in Str/ort mice [32,36]. Genetically modified mice show that many genes are involved in OA development. More than 18 different genetically different mouse strains were used in OA preclinical study as early as 1956 [37]. The ease of genetic engineering in mice has greatly advanced our understanding of OA pathology. The genetically engineered mice can be broadly classified into four broad categories dependent on gene or genes that are manipulated: cartilage matrix degradation, terminal (hypertrophic) chondrocyte differentiation or apoptosis, inflammation or synovitis, and bone turnover [38]. For example, a mutation in collagen or collagenase leads to the development of OA at a different age, confirming the crucial role of cartilage in OA development [39-41].
Non-surgical-induced OA: This is caused by intra-articular injection of chemicals or proteinases including iodoacetate, papain, or collagenase, which induces joint swelling and OA histological changes at greater rates of speed and severity [42-45]. Although non-surgical-induced OA has less variation among animals and fast development of OA-related pain in pre-clinical OA research,its pathogenesis does not really represent the development and progression of human OA. As a result, it is not the ideal option for OA pre-clinical study and often requires another model for validation.
Limitations of murine models of OA: Preclinical mouse OA models greatly advance our understanding of OA pathogenesis and provide a valuable tool to evaluate new OA drugs. However, a major limitation of preclinical models is that the anatomy and physiology between mice and human is not the same. For instance, human do not have the MMTL ligament. Moreover, unlike a variety of imaging modalities available to OA patients, preclinical moue OA models do not have an inexpensive and efficient imaging modality to monitor disease progression longitudinally. The primary outcome measurement of murine models of OA is structural damage or erosion of articular cartilage. The assessment of severity of OA relies on endpoint histology parameters such as the OARSI scoring and cartilage area [46]. Using endpoint assessment as primary outcome measurement greatly increases the number of experimental mice and associated cost. More importantly, the OA animal models vary in terms of severity and progression, thus evaluating OA progression with an in vivo imaging modality before the endpoint outcome measurement is beneficial. One of the most important symptoms of human OA, which can only be measured indirectly by parameters in mouse OA models.
Commonly used imaging modalities for human OA include X-ray, microCT (μ-CT) and magnetic resonance imaging (MRI) to observe changes in the anatomic feature, cartilage, subchondral bone, and synovium. These techniques have also been applied to murine OA models with limitations.
X-ray: Radiography is a commonly used tool in the diagnosis of OA in patients. In 1957, Kellgren and Lawrence established semi-quantitative assessments of knee OA on X-ray film. Known as the KL grading system, this uses 5 grades of osteophyte formation that include narrowness of joint space, subchondral bone sclerosis, and morphometry change of the articular surface [5]. Due to the heterogeneity of OA pathology, the occurrence of these X-ray-based biomarkers varies among individuals and grading of joint space narrowing and osteophyte is observer-dependent [47,48]. A quantitative assessment of joint space width to better characterize OA progression was developed around the 1990s and its sensitivity was validated with MRI imaging [49-51]. However, radiographic imaging is limited to the observation of bone tissue, cartilage loss, and deformation at a very late stage of the disease. Meniscus, synovitis, joint swelling that manifest in the early stages of the disease cannot be detected with radiography. Moreover, few studies utilize radiography on murine models of OA due to limited resolution of small animal X-ray [24]. Moreover, joint space narrowing in mice OA happens at end stage disease, where OARSI score is 5-6, whereas most OA studies are concerned with early stage disease in order to identify new therapeutic targets.
μ-CT: Subchondral bone remodeling is one of the characteristics of OA, including change in bone volume, growth plate morphology and increased bone turnover [52]. CT and high resolution CT is an X-ray based high-resolution imaging method optimal in evaluating subchondral bone plate morphology, thickness, trabecular pattern, osteophyte formation and calcification in the tendon [53,54]. Based on the utility of CT in clinic, laboratory μ-CT was developed and first used in a spontaneous OA mice model in 2004 to observe osteophyte, trabecular remodeling, subchondral bone plate thickening and sclerosis [55]. An ex vivo quantitative μ-CT method was later developed to evaluate the subchondral bone change in a collagenase-induced OA mice model [56]. McErlain et al. used in vivo μ-CT to measure volumetric bone mineral density in the subchondral area in rats with OA and found that they had lower bone mineral density four months after ACLT [57]. Botter et al. used in vivo μ-CT to evaluate cartilage damage and osteophytosis in a chemical-induced mouse OA model and found more cartilage loss, more osteophytosis, growth plate thinning, and increase in growth plate porosity at an early stage of OA, which could not be seen later [58]. In addition to assessing subchondral bone, μ-CT can also assess articular cartilage with phase-contrast imaging. Kotwal et al. used ionic contrast reagent Ioxaglate to detect the decrease of cartilage thickness in exercise-induced OA in mice ex vivo and validated their μ-CT findings with histological and biochemical methods [59]. Ruan et al. used contrast reagent osmium tetroxide for ex vivo contrast-enhanced μ-CT scanning in cruciate ligament injury and a DMM OA mouse model. They did 3D reconstruction to quantify cartilage volume, cartilage surface and bone surface area and validated results with histological scoring [60]. Lakin et al. further analyzed tibial cartilage glycosaminoglycan content in a collagenase-induced OA mice model ex vivo. They showed that glycosaminoglycan content measured with contrast-enhanced μ-CT correlated well with Safranin staining, validating their μ-CT findings [61]. It should be noted that although some ex vivo μ-CT imaging modality yields high resolution cartilage data, it is more similar to endpoint measurements such as histomorphometric analysis. This data should not be compared to data acquired from in vivo imaging. Moreover, since μ-CT is an X-ray based imaging modality it is not optimal for evaluating changes in soft tissues.
MRI: MRI is ideal for arthritic imaging due to its ability to distinguish the following joint soft tissues: cartilage, subchondral sclerosis, synovial fluid, osteophyte, ligament, patella position, and synovitis. Semi-quantitative standard, Whole-Organ Magnetic Resonance Imaging Scoring (WORMS) has been established for human knee OA, taking cartilage, marrow abnormality, bone cysts, bone attrition, osteophytes, compartment, menisci, ligaments and synovitis into consideration [7]. Ostergaard et al. found that synovial volume measured with MRI is highly correlated with the synovial hypertrophy scoring in RA patients, which is an indicator of inflammation of arthritis [62]. Our group used MRI to observe the progression of joint tissue damage in an RA mouse model and developed quantitative MRI-based biomarkers. These biomarkers include synovial volume and popliteal lymph node volume validated with μCT and histology analysis [63,64]. MRI is also applied to small animal OA models to evaluate cartilage and soft tissues. Munasinghe et al. used MRI to monitor joint structures of exercise-induced OA in 3-, 7-, and 12-month old Str/Ort mice. They found thickening of the patellar tendon, deformity, and sclerosis in 7-12 month-old OA mice, which was more severe in males than in females [65,66]. Goebel et al. assessed changes of femorotibial cartilage in rat knees that received ACL or sham surgery by 3D MRI at days 8, 14, 21, 40 and 60 post-surgery. Results indicated that mean cartilage thicknesses in OA knees decreased at an early phase (day 8, day 14) compared to sham knees and remained relatively stable thereafter. The histological correlation was significant only in untouched healthy cartilages [67]. These studies demonstrate the ability of MRI technique in assessing OA-related changes in femorotibial cartilage volume/thickness. Although MRI has been established as a useful tool to measure changes in soft tissues (i.e. synovial volume) it is very expensive. MRI data processing is time-consuming and requires specially trained operators. Seeking another imaging modality for mouse OA study is therefore encouraged.
Single photon emission computed tomography (SPECT) and positron emission tomography (PET): Scintigraphy is an imaging module in which radioisotopes are attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals). The drugs are injected systemically and the emitted radiation is captured by external detectors (gamma cameras) to form 2D images. It is widely used for tracing bone tumors and inflammation [68,69]. In a rabbit ACL OA model, 99mTc-J001 was injected into the animals, internalized by macrophages and detected by scintigraphy, which showed that OA knee joint has higher scintigraphic signals. This showed higher internalization of 99mTc-J001, suggesting higher bone metabolism in OA [70]. SPECT also uses gamma cameras to capture emitted radiation from radioisotopes to generate 3D images [71]. SPECT was used with μ-CT to monitor bone turnover in a MLI-induced OA model in rats. While μ-CT was used for anatomic localization, SPECT showed increased subchondral turnover in OA joints when compared to healthy joints [53]. Similarly, radiolabeled tracer such as 18F-fluoride and 18F-FDG, are used to assess metabolism in PET imaging. Franc et al showed that both acute and chronic phases of arthritis have higher PET signal with 18F-AraG tracer in a mice adjuvant arthritis mouse model [72]. However, radioisotope methods had a resolution of 1-2mm, which is insufficient to depict the anatomy of mice knee joint [47].
US is to send pulses of US into tissue and then record sound echoes from the tissue as B-mode images or color Power Doppler (PD) signals, both of which can be reconstructed into a 3D volume measurement. Different tissue types have different echogenic properties, depending on their location relative to the surface, density, and fluid content [73]. B-mode US image arises from the coherent interaction of random scatterers within a resolution cell when a certain anatomical region is scanned [73]. Two-dimensional images of the tissue are acquired with B-mode imaging, allowing researchers to inspect the anatomy of the tissue and navigate the identification of the region of interest for Power Doppler imaging. The Doppler Effect is named after Austrian physicist Christian Doppler in 1842. It describes the change in frequency of a wave (or another periodic event) for an observer moving relative to its source. Doppler ultrasound permits real-time viewing of blood flow through a blood vessel, and this method has been used to evaluate the major arteries and veins of the body, the heart, and in obstetrics for fetal monitoring. Power Doppler is one of Doppler ultrasound techniques with high sensitivity that could also detect the direction of flow.
Rheumatologists started using US imaging to evaluate joint and soft tissue in the 1990s [74,75]. Since then, utilization of US in RA clinic has been shown to be beneficial to RA diagnosis [76-79]. The 2010 new classification criteria for RA issued by American College of Rheumatology (ACR) and European League against Rheumatism (EULAR) focused more on inflammatory changes in the joint, which is optimal for US imaging. In 2013, EULAR issued ten recommendations for RA imaging in clinical management, nine that involve US, which popularized its use in in RA clinics [80-83].
US imaging in human OA joints: OA pathology includes inflammatory changes in the joint, providing the rationale to use US to monitor OA progression and its responsiveness to therapy. In fact, the utilization of US imaging grows rapidly in OA clinics because structural abnormalities detected by the US are commonly correlated with clinical endpoints [84-87]. US are more sensitive in detecting osteophytes and cartilage changes during arthroscopy [88-90]. To avoid confusion with terminology used in traditional radiology and MRI findings, the term “ultrasonographic” is added prior to the observed physical or pathological anatomy structures. US-based biomarkers for OA have been developed according to findings in several clinical studies [9,91-95]. For instance, synovial thickness>=4mm is referred to as ultrasonographic synovitis in OA in an EULAR clinical study. This is validated with X-ray [91,92]. Suprapatellar or synovial effusion depth>=4mm is diagnosed as ultrasonographic effusion [9,93]. In 2016, the reliability of US biomarkers on US findings from 13 patients with early knee OA was evaluated by 11 US experts in the Outcome Measures in Rheumatology (OMERACT) US Task Force on knee OA. A semi-quantitative scoring system was used to measure ultrasonographic synovitis, osteophytes, cartilage and meniscal damage. The results moderate to good intra- and inter-observer reliability scores for synovitis and fair to good of intra- and inter-observer reliability scores for cartilage or meniscal damage and osteophytes range, respectively. This suggests that using a standardized protocol and semi-quantitative US scoring of pathological changes in knee OA can be reliable [96].
US imaging system for healthy mouse knee: To determine whether US can be used to evaluate pathology of knee joints in mice,we examined a WT mouse knee with small animal US system. Although various small animal ultrasound systems are commercially available, we used Vevo770 (VisualSonics, Toronto, Canada) because the RMV704 transducer had a center frequency of 40 MHz, with an axial resolution of 40μm and a lateral resolution of 70μm, which is most appropriate for the observation of mouse knee joint (Table 1). The highest center frequency of the L38-22v CMUT transducer from VeraSonics is 28MHz, which is not sensitive enough for the observation of mouse knee joint. On the other hand, the center frequency of ultrasound biomicroscope systems could be as high as 275 MHz, allowing the observation at a cellular level, which is also not appropriate for joint imaging. In brief, anesthetized mice are placed in a supine position with their knees flexed over a customized mould to an approximately 135o angle. US gel is applied between the skin and the 704 US scan head. The knee joint is scanned in a vertical direction. US detection presents as a triangular area underneath of skin, which can be delineated by a hyperechoic line of femoral and tibial articular cartilage (Figure 2). Histology of the same region revealed that it is composed of the patellar ligament, soft tissues including synovium, fat pad, soft tissue portion of the meniscus, and empty (synovial) space (Figure 3A). Histology observation cannot be precisely matched to US findings due to the following reasons: 1) US could detect fluid in the synovial space (Figure 3B). However, histological analysis requires fixation, dehydration, and sectioning, which results in the loss of joint fluid. 2) US wave is completely reflected at calcified bone surface. Anything beneath calcified bones is not detected in US images. Thus if calcification occurs in the synovium or the meniscus, anything beneath calcification is not detected. 3) US detection reflects the 3D shape of joint soft tissues, while histology only reflects a 2D image of a joint at certain embedded rotation. Thus, ultrasound findings in the knee joint is defined as US synovial volume (USSV), which includes the space below patellar ligament and above mature bone tissue of the femur, tibia, and meniscus. This differs from synovial space in an H&E-stained section in which normal knee joints only contain the space between the meniscus and articular cartilage, which is very small. However, USSV is larger in a normal joint that contains various soft tissues and fluid.
US imaging in mouse RA knee: Dr. Schwarz’s group used US to examine joints of TNF-Tg mice, a mouse model of RA (11), because US is frequently used in RA clinic due to its nature of severe inflammation. They detected significantly increased USSV in TNF-Tg mouse joints, which correlates strongly with synovial volume measured with CE-MRI (63). Furthermore, the RA joints also have increased PD volume (PDV) measured with PD-US, which represents the volume of blood vessels. The PD signal is further validated by immunohistochemistry staining showing CD31 positive staining at the same site.
US in mouse OA joints: Compared to RA, OA joints have a much lower degree of inflammation and perhaps other soft tissue changes, making US detection more difficult. Spriet et al. first observed femoral cartilage damage in a rabbit ACLT OA model in vitro with B-mode US biomicroscopy. They semiquantitatively graded their US finding and validated US grade with histology grade, proving the potential of using US to monitor OA progression in small animals [97]. However, translating US biomicroscopy technique requires opening joint capsule to expose joint surface, which is invasive and cannot be used longitudinally.
in vivo US imaging protocol in an MLI OA mouse model (Figure 4). B-mode US at 4 and 11 weeks post-MLI on the same joints was performed. At the 4 week point, USSV was 2.76 +/- 0.54 (mm3) in MLI joints compared to 1.96 +/- 0.26 (mm3) in sham joints (p=0.005, un-paired t-test). At the 11 week point, USSV increased to 4.7 +/- 0.64 (mm3) in MLI joints while USSV remained unchanged (1.92 +/- 0.24 mm3) in sham joints (p=2.43E-07, un-paired t-test). More importantly, USSV was markedly increased at the same joint that received MLI (p=6.66E-06, paired t-test), but not in joints that received sham operation (p=0.84, paired t-test). A significant change in MLI joints (73.14 +/- 17.56% in MLI vs. 0.07 +/- 18.06% in sham, p=1.8E-04, un-paired t-test) was detected when the percentage change of USSV between 4 and 11 weeks was calculated. An average of USSV in normal knee joints of 30 mice is 1.97 +/- 0.17 (mm3). Thus, a standard normal USSV in adult mice is below 2.15 mm3. Increased PDV was found in mouse knees that received Hulth-Telhag surgical procedure that combines MLI and ACLT, causing severe cartilage loss [12,98].
Based on these preliminary findings, US could be used to monitor OA disease progression in mouse PTOA models. It can detect early soft tissue changes prior to cartilage loss and allow tissue changes at the same joint to be compared [22]. Thus, quantitative USSV by US in OA joints have the following advantages 1) providing a 3D volumetric measurement of peri-articular soft tissue in real time; 2) randomizing experimental groups in intervention studies; 3) reducing the number of mice since the changes of USSV can be compared before and after the treatment at the same joint, and 4) enabling researchers to adjust the duration of treatment according to longitudinal USSV.
By using the Vevo770 system with a US frequency of 40 MHz, our group has gained experience in US mouse knee joint and peri-articular soft tissues. However, US in mouse OA models also has its limitations. First, OA is a chronic disease with much less inflammation than RA. US may not be able to detect mild inflammatory changes and blood flow/vessels in peri-articular soft tissues of an OA joints. Second, the most common form of OA is an aging-related degenerative disease, which differs from PTOA used in our study. Soft tissue inflammation occurs in age related OA in mice has not been well studied, partially due to the lack of a non-invasive longitudinal imaging modality. Third, US imaging of knee joint cannot distinguish fine tissue structures detected by histology and cannot be used to replace histological analysis. Finally, imaging and data analysis vary among individual researchers who are sectioning the region of interest from each image frame and orientation of the knee joint. As a result, one single researcher has to complete all the US imaging sections and data analysis for a given experiment. To reduce the variation and increase scientific rigor, the US imaging and sectioning of the joint space volume should be standardized by recognition of anatomic landmarks in the knee joint, and US data should be analyzed blindly (and preferably) by a different researcher.
Some of these limitations are due to intrinsic nature of OA mouse models, and others may be related to the resolution of the Vevo770 system. The latest US machines such as Vevo3100 imaging system has better resolution The US frequency in the Vevo3100 imaging system is increased by 1.75 fold (70 MHz vs. 40 MHz in the Vevo770 system), greatly increasing the image resolution and enabling recognition of individual ligaments and meniscus. Another way to improve resolution is to use US-guided approach by injecting US contrast reagent into the knee joint. Furthermore, with enhanced resolution in the new imaging machine, we might be able to detect blood flow change in OA synovial soft tissues by PD-US.
Photoacoustic US has seen rapid growth as a biomedical imaging technique. In this imaging modality, endogenous energy is absorbed by the tissue and results in thermoelastic expansion of the tissue, which can be detected by an US transducer. Tissues with different blood flow and oxygenation status absorb heat differently, thus photoacoustic has been used in tumor diagnosis and other diseases [99]. Mice OA joints have thickened synovium and increased blood flow due to chronic inflammation, thus photoacoustic ultrasound shows great potential to monitor inflammation when combined with B-mode and Doppler ultrasound. However, it should be cautioned that since a healthy knee joint is not rich in blood vessels or soft tissues, it might be challenging to establish a basal level of photoacoustic signal. Moreover, typical OA develop milder synovitis than RA, thus it is more applicable to establish a robust photoacoustic protocol in the latter to determine whether this technique can detect small changes in blood flow and oxygenation status.
US imaging represents a promising alternative for OA imaging, especially in terms of observing synovial soft tissues during disease progression. Advantages of US imaging include no radioactive exposure, relatively low cost, easy to learn and less time-consuming when compared to MRI. With advancing imaging techniques, it is likely that in vivo US imaging may achieve higher resolution and render more information from a live mouse knee joint.
This work was supported by grants from the National Institute of Health, USA (AR63650 and AR069789) and NYSTEM, USA (CO-29548) to Lianping Xing.
Xi Lin declares that she has no conflict of interest. Lianping Xing declares that she has no conflict of interest. All the animals used in this study were approved by Animal Care and Use Committee of University of Rochester, USA. This article does not contain any studies with subjects.
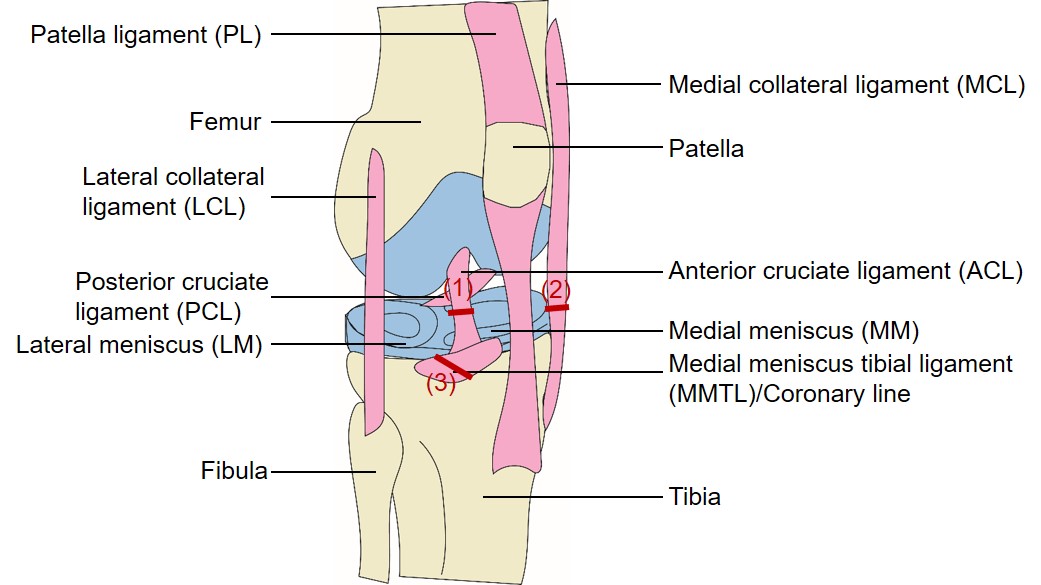 |
| Figure 1: A mouse knee joint and surgery procedures that induce posttraumatic OA. Scheme of a mouse knee joint. Potential targets of posttraumatic OA model are indicated in numbers. (1) ACLT (anterior cruciate ligament transaction): transection of ACL. (2) MLI (meniscal-ligamentous Injury): transection of MCL plus removal of medial meniscus. (3) DMM (destabilization of the medial meniscus): transection of MMTL. |
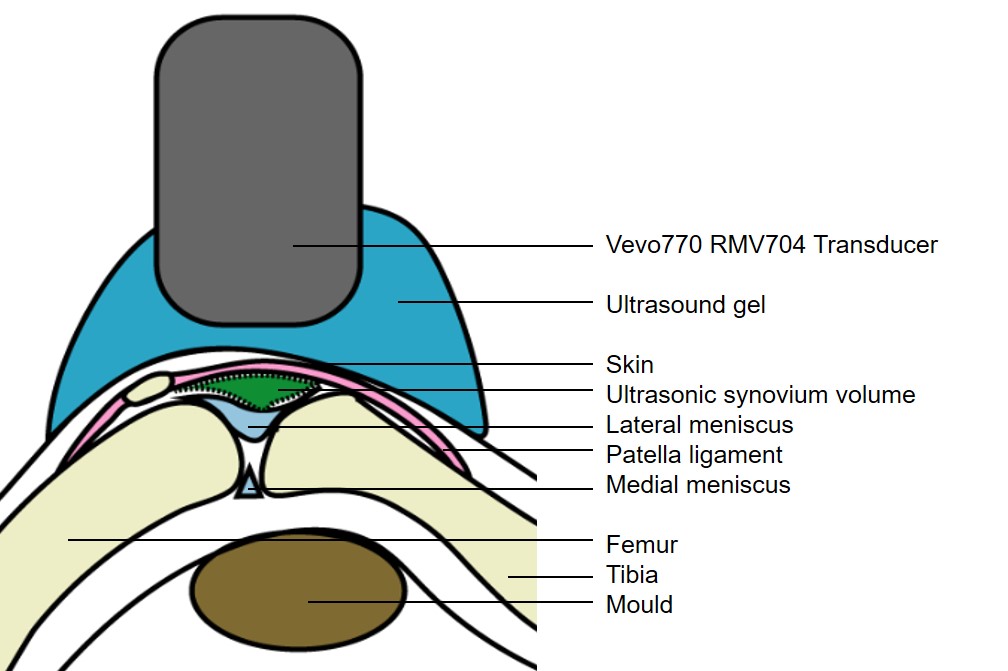 |
| Figure 2: Ultrasound imaging detects OA progression. Knee joint is bent at an angle with a mould underneath. Centrifuged ultrasound gel is sprayed on the skin surface after completely removing fur with depilatory cream. The Vevo ultrasound RMV704 transducer is lowered and immersed in the gel to acquire 2D B-mode ultrasound images. For 3D data acquirement, the scan head moves in a set range vertical to the paper |
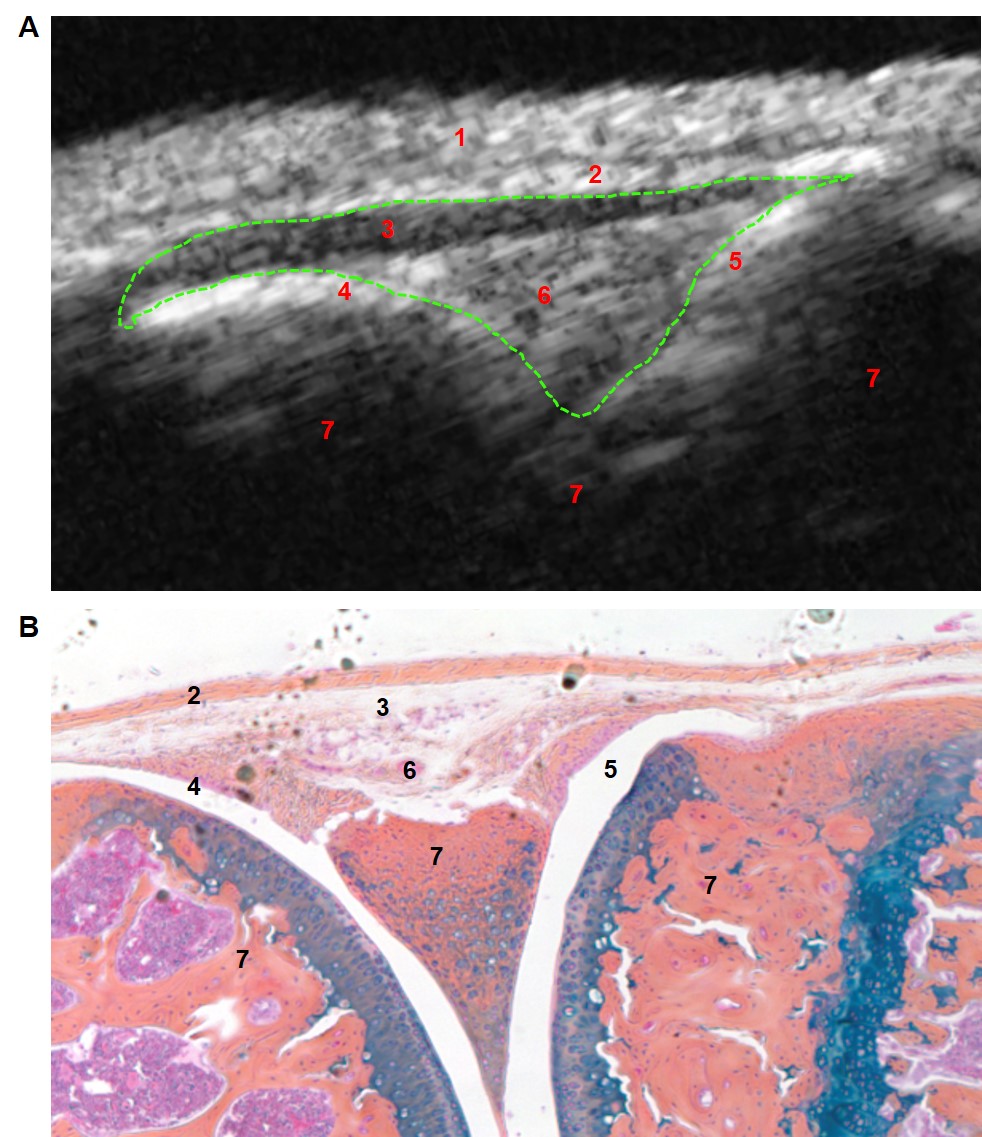 |
| Figure 3: Ultrasound imaging of C57Bl/6 male mouse knee at 3 month of age. (A) B-mode US imaging of the knee joint. The segmentation of US synovial volume is outlined by green USSV. Numbers indicate structures corresponding to those in histology. 1: Skin and hair. 2: Patellar ligament. 3: Fat pad. 4&5: Synovial space. 6: Connective tissue. 7:Calcified bone tissue. (B) Alcian blue/Orange G staining of the same knee joint. |
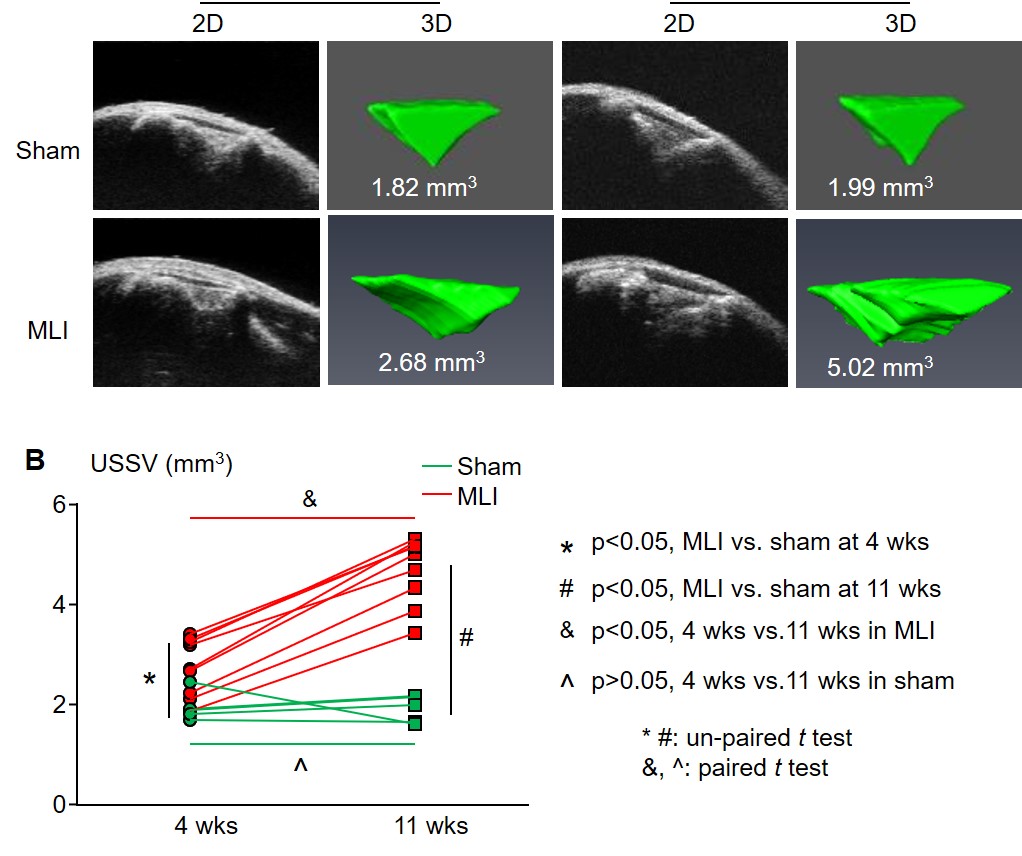 |
| Figure 4: Ultrasound imaging detects OA progression. KWT mice (male, 10-week-old) received MLI surgery on the right knee (N=9) and sham surgery on the left knee (n=5). Mice were subjected to B-mode ultrasound scanning followed by 3D reconstruction and USSV measurement with Amira four and eleven weeks after MLI. (A) Representative ultrasound images of sham (green) and MLI (red) surgery mice 11 weeks after MLI. (B) USSV increased in MLI but not sham surgery group. |
Manufacturer |
Transducer |
Center frequency (MHz) |
Axial resolution (µm) |
Lateral resolution (µm) |
||||
|---|---|---|---|---|---|---|---|---|
Verasonics |
L38-22v CMUT |
28 |
- |
- |
||||
Visualsonics Vevo3100 |
RMV704 |
60 |
40 |
70 |
||||
Visualsonics Vevo3100 |
MX700 |
70 |
30 |
65 |
||||
Ultrasound Biomicroscopes1 |
LN_300 |
275 |
6.2 |
6.4 |
||||
1 Fei, C., Chiu, C. T., Chen, X., Chen, Z., Ma, J., Zhu, B., Shung, K. K., and Zhou, Q. (2016) Ultrahigh Frequency (100 MHz–300 MHz) Ultrasonic Transducers for Optical Resolution Medical Imagining. Scientific reports 6, 28360 |
||||||||
Table 1: Comparison of different ultrasound system. |
||||||||






































