Top Links
Journal of Nutrition and Health Sciences
ISSN: 2393-9060
Chemical Constituents of the Fruits of Crataegus dahurica and the Antihyperlipidemic Activity in HepG2 CellsAbstract
Copyright: © 2020 Gao Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
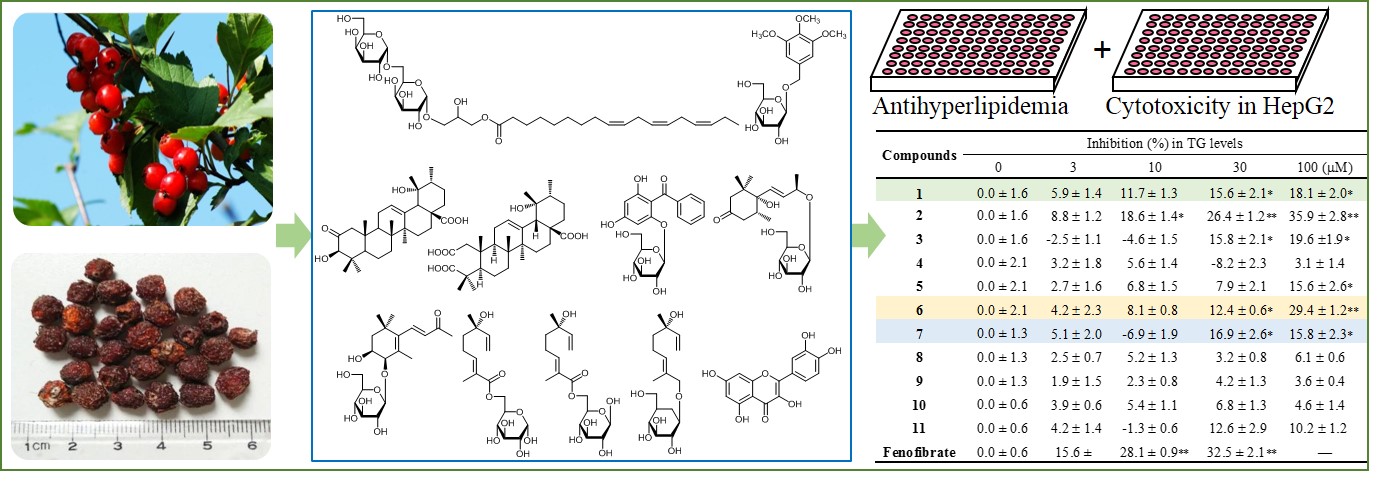
The fruit of Crataegus dahurica Koehne ex C.K. Schneid is an edible wild fruit and herb for the treatment of various diseases. However, few studies were conducted to search the chemical constituents of the edible herb. In the present study, a new glycoglycerolipid (1), together with ten known compounds, 2-oxo pomolic acid (2), cecropiacic acid (3), nikoenoside (4), garcimangosone D (5), ampelopsisionoside (6), saussureosides B (7), 6-O-[(2E, 6S)-2, 6-dimethyl-6-hydroxy-2, 7-octadienoyl]-α-D-glucopyranoside (8), 6-O-[(2E, 6S)-2, 6-dimethyl-6-hydroxy-2, 7-octadienoyl]-β-D-glucopyranoside (9), betulalbuside A (10), quercetin (11), were isolated from thefruits of C. dahurica with various chromatographic techniques. Their structures were determined by extensive spectroscopic data analysis including 1D and 2D NMR and HR-ESI MS and comparison with previous literature. The inhibitory activity on TG accumulation in HepG2 cells was assayed to evaluate the antihyperlipidemic activity. The results showed that compound 6 significantly inhibited the accumulation of TG in cells without inducing cytotoxic activity. In addition, compounds 1 and 7 also exhibited the moderately inhibitory activity on TG in cells. The results in this present study would be helpful to further exploration and utilization of the edible herb.
Keywords:Crataegus dahurica Koehne ex C.K. Schneid; Edible Herbs; Glycoglycerolipid; Chemical Constituents; Antihyperlipidemic Activity
Crataegus dahurica Koehne ex C.K. Schneid, belonging to Rosaceae family, mainly distributed in northeastern provinces and Great Xing’an Mountains areas in China, as well as in the Far East region of Russia. The fruits of C. dahurica is not only as a wild food eaten by Oroqen people, a nomadic minority in Northeast China, but also used as an herb for the treatment of dyspepsia, hepatitis, and hyperlipidemia, etc [1,2]. However, no systematic chemical constituents and pharmacological activities of the wild edible and medicinal fruit has been reported except our previous study [3]. In the continuous research on the chemical constituents and biological activities of this edible herb, a new glycoglycerolipid together with ten known compounds were isolated and characterized, and the antihyperlipidemic activity in HepG2 cells was also evaluated. In this paper, we report the isolation and the structure elucidation of these compounds and their antihyperlipidemic activity in vitro. The findings in this study will be helpful to further utilize and develop this wild fruit as a dietary supplement or potential medicine.
1H and 13C NMR spectra were measured on a Bruker Avance III-400 spectrometer (Bruker, Germany), with tetramethylsilane (TMS) for the internal standard. Chemical shifts were reported in units of δ (ppm) and coupling constants (J) were expressed in Hz. IR spectra were recorded on a Bruker Tensor II spectrometer. The purification was conducted on a medium pressure system (PrepChromaster 6000plus, Kezhe, China) and a semi-preparative HPLC system (PuriMaster 5000, Kezhe, China). The silica gel (Qingdao Haiyang Chemical Co., Shandong, China), Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Sweden) and ODS (Merck Co., Germany) were used in the isolation and purification of the compounds. A triglyceride detection kit was obtained from Nanjing jiancheng bioengineering institute (Nanjing, China) and the absorbance measurements of triglycerides were conducted on a multiplate reader (SuPerMax 3100, Kezhe, China). The other chemicals and reagents were bought from Sigma-Aldrich (Sigma, St. Louis, MO, USA).
The fruits of C. dahurica were collected in August 2015, Tongbei, Heilongjiang province and identified as the previously reported [3]. A voucher specimen (MLN150816) has been deposited in the herbarium of the School of Pharmacy, Health Science Center, Xi’an Jiaotong University. And the CdME and the fractions were prepared according to the method described by Wang et al. [3].
The n-BuOH fraction (115.7 g) of C. dahurica was fractionated to thirty-six fractions (Fr.Bu1~Fr.Bu36) by silica gel (200-300 mesh, 1000 g) CC eluting with CHCl3/CH3OH (1:0, 80:1, 50:1, 20:1, 10:1, 5:1, 2:1, 0:1, v/v). Fr.Bu3 was further separated by silica gel CC eluting with PE/acetone (10:1, v/v), the sub-fractions were purified by semi-preparative HPLC with 80% MeOH in 0.5% acetic acid as the mobile phase to afford the compound 1 (10.3 mg), compound 2 (16.9 mg) and 3 (5.2 mg). Compound 4 (11.2 mg) was obtained from Fr.Bu13 which was separated by Sephadex LH-20 CC (methanol) and semi-preparative HPLC (30% methanol in 0.5% acetic acid). Fr.Bu14 was further separated to fourteen subfractions (Fr. Bu14-1 ~ Fr. Bu14-14) by ODS (212 g) CC eluting with CH3OH/H2O (15:85, 30:70, 45:55, 60:40, 80:20, 100:0, v/v). Fr. Bu14-6 to Fr. Bu14-8 were purified by semi-preparative HPLC with 35% methanol in 0.5% acetic acid to get compound 5 (4.7 mg), compound 6 (12.4 mg) and 7 (10.3 mg), respectively. Fr. Bu16 was divided into sixteen subfractions (Fr. Bu16-1 ~ Fr. Bu16-16) by ODS CC eluting with CH3OH/H2O (25:75, 34:66, 44:56, 57:43, 74:26, 100:0, v/v). Compound 8 (6.8 mg), 9 (4.1 mg) and 10 (5.1 mg) were purified from Fr. Bu16-8 with 40% methanol in 0.5% acetic acid through semi-preparative HPLC. The EtOAc fraction (36.2 g) was separated by silica gel (200-300 mesh, 500 g) CC gradient eluting with CHCl3/CH3OH (1:0 ~ 1:5, v/v) to yield twenty-three fractions (Fr.EA1 ~ Fr.EA23). Compound 11 (9.2 mg) was purified from Fr.EA16 by Sephadex LH-20 CC eluting with CH3OH/H2O (1:1, v/v).
1-O-(9Z, 12Z, 15Z-octadecatrienoyl)-3-O-[α-D-galactopyranosyl-(1→6)-O-α-D-galactopyranosyl] glycerol: White powder. HR-ESI MS m/z 699.35740 [M+Na]+ (calcd for C33H56O14Na, 699.35678); IR (KBr) nmax 3401, 1736, 1645 cm−1; 1H NMR (400 MHz, DMSO-d6): 3.96 (1H, dd, 11.2, 6.5, H-1), 4.04 (1H, dd, 11.2, 4.0, H-1), 3.80 (1H, m, H-2), 3.60 (1H, m, H-3), 3.55 (1H, m, H-3), 2.28 (2H, m, H-2¢), 1.50 (2H, m, H-3¢), 1.26 (2H, m, H-4¢), 1.25 (2H, m, H-5¢), 1.26 (2H, m, H-6¢), 1.30 (2H, m, H-7¢), 2.02 (2H, m, H-8¢), 5.34 (1H, m, H-9¢), 5.29 (1H, m, H-10¢), 2.77 (2H, m, H-11¢), 5.32 (1H, m, H-12¢), 5.31 (1H, m, H-13¢), 2.77 (2H, m, H-14¢), 5.28 (1H, m, H-15¢), 5.35 (1H, m, H-16¢), 2.03 (2H, m, H-17¢), 0.92 (3H, t, 7.5, H-18¢), 4.67 (1H, d, 3.4, H-1¢¢), 3.55 (1H, m, H-2¢¢), 3.28 (1H, m, H-3¢¢), 3.67 (1H, m, H-4¢¢), 3.54 (1H, m, H-5¢¢), 3.66 (1H, m, H-6¢¢), 3.43 (1H, m, H-6¢¢), 4.09 (1H, d, 3.6, H-1¢¢¢), 3.28 (1H, m, H-2¢¢¢), 3.60 (1H, m, H-3¢¢¢), 3.68 (1H, m, H-4¢¢¢), 3.61 (1H, m, H-5¢¢¢), 3.44 (1H, m, H-6¢¢¢), 3.50 (1H, m, H-6¢¢¢) ; 13C NMR (100 MHz, DMSO-d6): 65.5 (C-1), 67.4 (C-2), 66.5 (C-3), 173.0 (C-1¢), 33.5 (C-2¢), 24.5 (C-3¢), 28.64 (C-4¢), 28.56 (C-5¢), 29.0 (C-6¢), 28.5 (C-7¢), 26.7 (C-8¢), 129.9 (C-9¢), 127.6 (C-10¢), 25.2 (C-11¢), 127.9 (C-12¢), 128.0 (C-13¢), 25.1 (C-14¢), 127.0 (C-15¢), 131.5 (C-16¢), 20.1 (C-17¢), 14.2 (C-18¢), 99.5 (C-1¢¢), 72.9 (C-2¢¢), 70.5 (C-3¢¢), 68.1 (C-4¢¢), 68.9 (C-5¢¢), 70.4 (C-6¢¢), 104.0 (C-1¢¢¢), 73.1 (C-2¢¢¢), 71.3 (C-3¢¢¢), 68.4 (C-4¢¢¢), 69.6 (C-5¢¢¢), 60.6 (C-6¢¢¢).
The assay was performed according to the previous method with slight modification [4-14]. Briefly, HepG2 cells were seeded in 48-well plates (1´104 cells/well) in DMEM (high glucose). After incubation for 24 h, the medium was changed and the samples dissolved in DMSO were added into the wells. The cells with or without the samples were cultured for another 4 days (change the medium every two days). At the end of the incubation, the medium was aspirated, and the cells were sonicated in distilled water (150 mL/well). The concentration of TG in every well was measured by a TG kit. Fenofibrate as a reference compound was tested in this assay. After 5 days’ culture, cytotoxic activity was also assayed by adding 10 μL of MTT to each well and incubating for another 4 h. Then 200 μL of DMSO was added to each well to dissolve the formazan after the removal of the supernatant. The absorbance was measured at 570 nm on a multiplate reader (SuPerMax 3100, Kezhe, China).
The hydrolysis of compound 1 was conducted as the previous literature [15,16].
With multiple chromatographic methods, eleven compounds (Figure 1), including a new compound, were isolated from the EtOAc fraction and n-BuOH fraction of the fruits of C. dahurica. All of these compounds were measured for 1H and 13C NMR data. Compound 1 was obtained as a white powder. Its HR-ESI MS revealed a pseudomolecular ion peak [M+Na]+ at m/z 699.35740 (calcd. 699.35678) established the molecular formula of compound 1 as C33H56O14 which showed six degrees of unsaturation. Its IR spectrum displayed characteristic bands for hydroxyl, carbonyl groups and double bonds at 3401, 1736 and 1645 cm−1, respectively. The 13C-NMR spectrum of 1 displayed six signals of unsaturated double bonds carbons, together with six sp2 hybridization protons signals in 1H-NMR spectrum, indicating the presence of three double bonds in the molecule. In addition, a quaternary carbon at δC 173.0 in the 13C-NMR spectra demonstrated the presence of an ester carbonyl group. In the 1H-NMR spectrum of 1, two anomeric protons [δH 4.67 (1H, d, J =3.4 Hz) and δH 4.09 (1H, d, J = 3.6 Hz)] and their corresponding carbons (δC 99.5 and 104.0) together with multiple oxygenated carbons signals from δC 60.6 to δC 73.1 indicated that there were two sugar units. After hydrolysis of 1, the sugar was identified as D-galactose by co-TLC with the control of D-galactose. Additionally, two molecules of D-galactose liberated from the hydrolysate by GC analysis also confirmed the result. The configuration of D-galactose was determined asa-orientation by their coupling constants of the anomeric protons. In the 1H-1H COSY spectrum, the correlations of three protons connected to oxygenated carbons attributed by HMQC spectra, from H-1 (δH 3.96, 4.04, δC 65.5) to H-2 (δH 3.80, δC 67.4) and H-2 (δH 3.80, δC 67.4) to H-3 (δH 3.55, δC 66.5) suggested the presence of a glycerol moiety in the molecule (Figure S1). One of the galactoses was linked to the left position of the glycerol which was determined by the HMBC correlations from H-1¢¢ (δH 4.67) to C-3 (δC 66.5) and H-3 (δH 3.60, 3.55) to C-1¢¢ (δC 99.5). And the right position of the glycerol linked to the carbonyl group by the formation of the ester bond with the hydroxyl group of the glycerol, which was confirmed by the observation of the cross peak of H-1 (δH 3.96, 4.04) to C-1¢ (δC 173.0). After extensive analysis of the 1H-1H COSY and HMBC spectra of 1 (Figure S1), it was found that the carbonyl group was the carboxyl carbon of a long chain multiple unsaturated fatty acid. Compound 1 was hydrolyzed with 5% NaOMe-MeOH to give a methyl 9Z, 12Z, 15Z-octadecatrienoate identified by GC with an authentic sample. The another a-D-galactose linked to the 6 position of the inner galactose confirmed by the correlations in HMBC spectra of 1 from H-1¢¢¢ (δH 4.09) to C-6¢¢(δC 70.4) and H-6¢¢ (δH 3.66, 3.43) to C-1¢¢(δC 104.0). Therefore, compound 1 was determined as 1-O-(9Z, 12Z, 15Z-octa decatrienoyl)-3-O-[α-D-galactopyranosyl-(1→6)-O-α-D-galactopyranosyl] glycerol, which was a previously undescribed compound.
The structures of the known compounds were characterized based on the physicochemical properties and compare their data with those reported in related literature. These compounds were identified as 2-oxo pomolic acid (2) [4], cecropiacic acid (3) [5]. nikoenoside (4) [6], garcimangosone D (5) [7], ampelopsisionoside (6) [8], saussureosides B (7) [9], 6-O-[(2E, 6S)-2, 6-dimethyl-6-hydroxy-2, 7-octadienoyl]-α-D-glucopyranoside (8) [10], 6-O-[(2E, 6S)-2, 6-dimethyl-6-hydroxy-2, 7-octadienoyl]-β-D-glucopyranoside (9) [10], betulalbuside A (10) [11], quercetin (11) [12], respectively. All of these compounds were isolated from the fruit and the whole plant of C. dahurica for the first time.
All isolated compounds were subjected to evaluate the inhibitory activity on TG in HepG2 cells. And the results showed that compound 6 could significantly inhibit the accumulation of TG in cells without induction of cytotoxic activity, compared to the control group. Meanwhile, compounds 1 and 7 also moderately reduced TG concentrations in HepG2 cells after 4 days of co-culture (Table 1 and S1). In addition, as shown in Table1, although compound 2 and 3 also significantly inhibited TG accumulation in cells (inhibition rate: 35.9 ± 2.8% and 19.6 ±1.9% at 100 mM, respectively), however, the inhibitory activity on TG was induced mainly by their cytotoxic activity (Table S1). Actually, this kind of triterpenoids was frequently reported to exhibit cytotoxicity in different cell lines [13].
This work was supported by the grants of the Natural Science Foundation of China under Grant [number 81673564]; and the Natural Science Foundation of Shaanxi province under Grant [number 2019JZ-01, 2019JM-121].
1H-1H COSY, main HMBC correlations, NMR, HR-MS and cytotoxicity of compounds are available alongside Figures S1-S8, Table S1.
Figure 1: Chemical structures of compounds 1-11 |
Compounds |
Inhibition (%) in TG levels |
||||
0 |
3 |
10 |
30 |
100 (mM) |
|
1 |
0.0 ± 1.6 |
5.9 ± 1.4 |
11.7 ± 1.3 |
15.6 ± 2.1* |
18.1 ± 2.0* |
2 |
0.0 ± 1.6 |
8.8 ± 1.2 |
18.6 ± 1.4* |
26.4 ± 1.2** |
35.9 ± 2.8** |
3 |
0.0 ± 1.6 |
-2.5 ± 1.1 |
-4.6 ± 1.5 |
15.8 ± 2.1* |
19.6 ±1.9* |
4 |
0.0 ± 2.1 |
3.2 ± 1.8 |
5.6 ± 1.4 |
-8.2 ± 2.3 |
3.1 ± 1.4 |
5 |
0.0 ± 2.1 |
2.7 ± 1.6 |
6.8 ± 1.5 |
7.9 ± 2.1 |
15.6 ± 2.6* |
6 |
0.0 ± 2.1 |
4.2 ± 2.3 |
8.1 ± 0.8 |
12.4 ± 0.6* |
29.4 ± 1.2** |
7 |
0.0 ± 1.3 |
5.1 ± 2.0 |
-6.9 ± 1.9 |
16.9 ± 2.6* |
15.8 ± 2.3* |
8 |
0.0 ± 1.3 |
2.5 ± 0.7 |
5.2 ± 1.3 |
3.2 ± 0.8 |
6.1 ± 0.6 |
9 |
0.0 ± 1.3 |
1.9 ± 1.5 |
2.3 ± 0.8 |
4.2 ± 1.3 |
3.6 ± 0.4 |
10 |
0.0 ± 0.6 |
3.9 ± 0.6 |
5.4 ± 1.1 |
6.8 ± 1.3 |
4.6 ± 1.4 |
11 |
0.0 ± 0.6 |
4.2 ± 1.4 |
-1.3 ± 0.6 |
12.6 ± 2.9 |
10.2 ± 1.2 |
Fenofibrate |
0.0 ± 0.6 |
15.6 ± 1.2* |
28.1 ± 0.9** |
32.5 ± 2.1** |
— |
Each value is represented as mean ± SEM (n=3)
Table 1: The antihyperlipidemic activity of the compounds from the fruits of C. dahurica in HepG2 cell






































