Top Links
Journal of Materials Science and Nanotechnology
ISSN: 2348-9812
Structural, Elemental and Molecular Characterization of Normal And Osteoarthritic Human Articular Cartilage
Copyright: © 2017 Çayır T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
The articular cartilage overlying the bone consists of a network of collagen fibres. This network is essential to cartilage integrity, usually suffering damage in degenerative joint diseases such as osteoarthritis. We have been applying a number of techniques to study the bone-cartilage interface and of changes occurring in this with disease. The bone-cartilage samples with disease were investigated for their structural, elemental and molecular properties. The bone-cartilage samples with disease were characterized by scanning electron microscopy- energy dispersive X-ray spectroscopy (SEM-EDX) and Fourier Transform infrared spectroscopy (FTIR) and Fourier transform (FT) Raman spectroscopy. The energy dispersive X-ray (EDX) analysis confirmed nearly stoichiometric samples. The Raman spectra made it possible to monitor the changes in the main bone constituents: the mineral component with the apatite band at 960 cm−1, the organic component with the collagen amide III band at 1268cm−1.Present results have been obtained on sections of bone not displaying evidence of an osteoarthritic lesion and can be used as a baseline against which diseased bone can be compared.
Keywords: Bone-cartilage; Raman; SEM-EDX; FTIR
Bone is a complex tissue which integrates two basic components, organic (matrix) and inorganic (mineral) material [1]. Its high strength and fracture toughness is achieved by a unique architecture of organic and inorganic phases [2-4]. It contains nano sized mineral platelets (essentially carbonated hydroxyapatite), protein (mainly collagen type I), and water [3,5,6]. The matrix makes up 30-40% of the total bone material and consists of collagen and other non-collagenous protein sand lipids [6]. Around 85-90% of the total protein content is made up by collagen fibrils. The mineral substance is mainly a carbonated form of crystalline calcium phosphate with a total percentage in bone material of 60-70% [7]. Therefore, because of the structural complexity of bone, its healing is an extremely complex process which has been under extensive investigation for many years [8]. Articular cartilage has an extracellular matrix composed primarily of water, type II collagen fibrils and proteoglycans [9]. Histologically, articular cartilage has unique variations in its morphological structure and molecular composition across its tissue depth, which is commonly considered to comprise three sub-tissue zones based on local fibril orientation [10]. These three zones are (a) the superficial zone (SZ) where the collagen is oriented parallel to the articular surface, (b) the transitional zone (TZ) where the collagen is oriented rather randomly, and (c) the radial zone (RZ) where the collagen is oriented mainly perpendicular to the articular surface. The critical role of the collagen matrix in cartilage is to preserve the tissue integrity where any alteration of the collagen microstructure due to tissue lesions will inevitably disrupt the molecular environment, consequently modifying the mechanical properties of the tissue.
Osteoarthritis is a joint disease that affects most people in the middle and elderly ages. It's slow. The cartilage structure of the joint due to aging changes. Being overweight, fractures in cartilage due to falling or some other mechanical trauma, followed by damage to the menus and ligaments, narrowing of the joint space and new bone formation.
OA is the most common form of arthritis, with an average prevalence of 10-12% in various populations. It is the most common cause of disability and pain caused by musculoskeletal system in geriatric patients. OA affects 10% of the population in 55 ages; at the age of 75, this rate exceeds 50%. Prevalence and the severity of the disease are increasing with age. Age is thestrongest sign of OA in all joint regions. OA affects both genders, while the ratio of female to male is somewhat between 1.5: 1 and 4: 1 changing Table 1.
Several microscopic imaging techniques have been used to study the load-induced deformation of the collagen matrix in articular cartilage. For example, microscopic MRI (lMRI) has been successfully used to image the modification of the tissue morphology in intact tissue blocks based on the proton signals in water molecules [11]. Scanning electron microscopy can directly visualize the effect of mechanical loading on the organization of the collagen fibrils in cartilage matrix [12]. The technique of Fourier transform infrared (FT-IR) spectroscopy is a powerful tool to study the previously described changes in degenerative cartilage at the molecular level. Raman analysis is sensitive to molecular orientation, and discerning between orientation and compositional contribution is crucial as bone is comprised of mineralized collagen fibres with alternating orientation within successive lamellae. The capability of Raman microspectroscopic analysis to distinguish between structural and chemical changes in bone were reported, by exploiting the dual influence of composition and structure with in the sample on the Raman signal [13,14].
Two human femoral head cartilage specimens from the surgery of humans are reported in this paper. While one of the samples is of a osteoarthritic cartilage the other is a healthy cartilage. The healthy cartilage was due to a femoral head fracture. Prior to the sample analysis, there were no fixations, embedding or surface processing. This work is an effort to characterize the healthy and diseased bone-cartilage by using a combination of techniques such as scanning electron microscopy- energy dispersive X-ray spectroscopy (SEM-EDX), FT-IR and Raman spectroscopy. The aim of this study is to make comparison of structural, elemental and molecular properties of healthy and diseased bone-cartilage samples.
Human femoral heads from total hip replacement procedures were used, due to surgicalintervention in responsse to a degenerative joint disease.The femoral heads were sourced from the department of Orthopedics and Traumatologyat Kocaeli University(Turkey). Using a water-cooled diamond saw(Isomet 1000 Precision ) severalsections at certain thicknesses were cut perpendicular to the articular surface from the superior aspect of the femoral head with thicknesses ranging from 200μm to 300μm. These sections were subsequently soaked in distilled water to remove any bone marrow and loose particulate matter. Morphological and elementel investigations were conducted on a scanning electron microscopy (Zeiss Supra 50 VP model SEM-EDX) with an acceleration voltage of 25 kV.Absorbance FT-IR spectra were obtained using a Thermo Scientific spectrometer in the range of 4000-400 cm−1 using KBr pellets at room temperature. The resolution was 2 cm−1, and the number of scans was 30.
An inViaReflex™ and an inViaQontor Raman microscope (Renishaw plc, Wotton-under-Edge, UK) were used. The spectrometers were configured with a diode laser excitation (785 nm) with the laser power of 300 mW,a 600 g mm-1 grating (Reflex) / a 1200 g mm-1 grating (Qontor),a motorized XY stage and a charge coupled device (CCD) detector. A line-shaped laser output was focused onto the sample through a microscope using a ×20 microscope objective.
The surface morphology and elemental analysis of the healthy and diseased femoral heads were characterised by scanning electron microscopy and enery dispersive X-Ray spectrometer. Figure 1 shows the SEM images of the osteoarthritic femoral head cartilage sample and healthy cartilage (femoral head fracture) sample.
Figure 1 shows that femoral head fracture cartilage have a smooth surface structure. The cartilage surface contains the presence of lacuna, chondrocytes and the chondroblasts structures. For the osteoarthritic femoral head cartilage, the level of surface deformation increased. Because of deterioration cartilage surface has occurred as the disease progresses. Consequently, the surface properties significantly, changed due to with cause deformation and diseases.
Figure 2 shows the EDX data of the osteoarthritic femoral head cartilage sample and healthy cartilage (femoral head fracture) sample. Figure 2 shows that healthy and diseased cartilage samples have elements such as Ca, P, S, C, etc. It is evident that healthy bone cartilage sample has more amount of elements such as calcium, phosphorus, etc., than the diseased cartilage.
Figure 3 shows the RAMAN spectra of the osteoarthritic femoral head cartilage sample and healthy cartilage (femoral head fracture). Surface StreamLine images were obtained in an area of 773.9μm x 248.5 μm, using 90 mW laser power, 4 seconds/line acquisition time, and a 7.1 μm step size.
The human osteoarthritic cartilage tissue section is shown in Figure 4. The data collection points are indicated by stars and numbered as: 1-) the cartilage, 2-) bone-cartilage interface, 3-) bone section, close to the interface and 4-) bone middle section.
Figure 5 shows the Raman spectra from the four different regions that are indicated in Figure 4. The prominent bands are labeled. The υ1 phosphate stretching vibration at 960 cm−1 is the strongest marker for bone mineral. The broader bands are assigned to amide III (~1239 and 1268 cm−1), the C–H bending mode (~1450 cm−1), and amide I (~1668 cm−1). The amide I at ~1668 cm−1, and amide III ~1239 and 1268 cm−1 peaks are mainly due to the presence of collagen, while the C–H bending band at ~1450 cm−1 is present in both collagenous and noncollagenous organic moieties. When the spectra are compared Number 1 through Number 4, from the interface to the bone middle section, the relative intensity of the phosphate stretching vibration is increased as a result of increase in the mineral content. Whereas, the relative intensity of the band vibrations that are responsible from organic content of the tissue section is decreased. The osteoarthritic femoral head cartilage sample was analyzed by Live Track StreamLine and the image of the sample illustrated in Figure 6.
The spectrum displays mainly signals that are coming from the protein content, such as tryptophan, C-C protein, proline, hydroxyproline(874 cm−1), phenylalanine (1003 cm−1 and 1030 cm−1), amide III (collagen type I, 1239 cm−1 and 1268 cm−1), CH from protein(1450 cm−1) and amide I (collagen type I, 1668 cm−1). Besides protein signals, the glycosaminoglycan and hydroxyapatite (PO43-) vibrations are also evident in the spectrum. The hydroxyapatite signal scores are relatively low values compared with the bone tissue sections.
Figure 7,8 shows FT-IR absorbance spectra of the samples. Two samples were characterized by use of FTIR spectrometry (Thermo Scientific, Erzincan, Turkey) as described. Data were collected in the absorption mode between 4000 and 400cm−1, with resolution 2 cm−1. We used previous assignments of absorption bands. For healthy cartilage regarding carbonated apatite[CA;Ca10(PO4)6(OH)x(CO3)y], the υ1 and υ3 P O stretching vibration modes were measured at 723 cm−1 and 1163 cm−1 respectively, and the O-P-Oυ4 bending mode corresponded to the doublet at 531cm−1. Regarding m-CPPD, O-P-O bending was recorded at 514 and 500cm−1.Amid I, Amid II and Amid III infrared bands were measured at 1740cm−1, 1464-1377 cm−1 and 1201 cm−1 respectively. These values change for diseased cartilage. For healthy cartilage regarding carbonated apatite [CA;Ca10(PO4)6(OH)x(CO3)y], υ3 P-O stretching vibration mode was measured at 1026cm−1, and the O-P-O υ4 bending mode corresponded to the doublet at 517cm−1. Amid I, Amid II and Amid III infrared bands were measured at 1747-1646 cm−1, 1540-1457 cm−1 and 1338-1200 cm−1 respectively.
We used SEM-EDX, FT-IR and RAMAN spectroscopy to characterize the healthy and diseased bone-cartilage samples. SEM technique is a useful tool to investigate the localization of specific proteins within the cartilaginous matrix and also within mineralized tissue. The technique provides ease of visualization of the location of specific collagens within cartilage as well as mineralized tissue. Due to the clear and definitive labeling that can be seen when using backscattered electron imaging, the technique of SEM can be used to quantify amounts of different collagens present in cartilage and mineralized tissue.SEM results show that the surface properties changed significantly due to deformation and diseases. It was found quantity of basic elements of the diseaded bone-cartilage sample decreased. RAMAN spectrum displays mainly signals that are coming from the protein content, such as tryptophan, C-C protein, proline, hydroxyproline. The Raman spectra from the four different regions that are indicated. The prominent bands are labeled. The υ1 phosphate stretching vibration at 960 cm−1 is the strongest marker for bone mineral. The mineral and organic structures obtained in cartilage, interface, bone and middle bone sections differ. The results obtained FT-IR analysis through also suggests disruption of bone cartilage structure. The results reveal that there are structural, elemental and molecular differences in healthy and diseased cartilages.
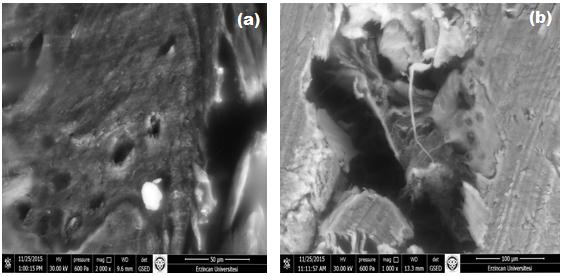 |
| Figure 1: SEM images of (a) healthy and (b) diseased cartilage samples |
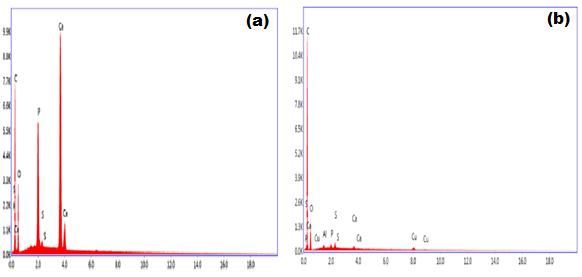 |
| Figure 2: EDX data healthy and diseased cartilage samples |
 |
| Figure 3: White light image of healthy and diseased cartilage. The yellow box indicates the Surface Stream Line imaged area/ The white box indicates Surface Stream Line analysed area |
 |
| Figure 4: White light image of a healthy cartilage section. The stars indicate where point spectra were acquired |
 |
| Figure 5: Raman spectra acquired from the locations marked by the stars in Figure 4 |
 |
| Figure 6: White lightimage of the osteoarthritic femoral head cartilage sample, as seen in Figure 3. The white box indicates the Surface Stream Line analysed area. At the cross hair position a single spectrum is acquired. An example spectrum from the cross hair position shown in Figure 6 |
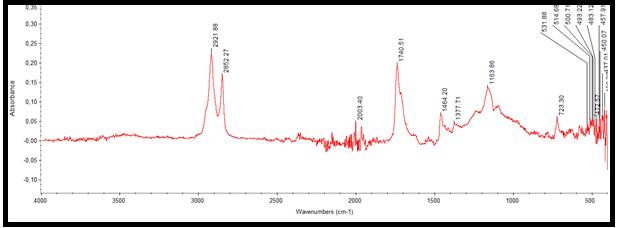 |
| Figure 7: FT-IR spectra of healthy cartilage samples |
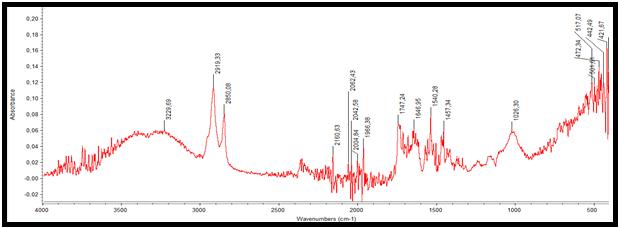 |
| Figure 8: FT-IR spectra of diseased cartilage samples |
Osteoarthritis |
Aging |
|---|---|
Cartilage hydration ↑
|
Cartilage hydration ↓ |
Proteoglycan concentration ↓ |
Proteoglycan concentration ↔ |
Collagen density ↓ |
Collagen density ↔ |
Chondrocyte proliferation ↑
|
Chondrocyte proliferation ↔ |
Metabolic activity ↑
|
Metabolic activity ↔ |
| Table 1: Relationship between Aging and Osteoarthritis | |






































