Top Links
Journal of Hematology and Blood Disorders
ISSN: 2455-7641
Correlation of Hemostatic Parameters with Poly (ADP-ribose) Polymerase-1 (PARP-1) Polymorphisms, Mutations, Laboratory, and Clinical Characteristics in 114 Patients with Philadelphia-Negative Myeloproliferative Neoplasms
Copyright: © 2021 Giannakopoulou N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Patients with Philadelphia-negative myeloproliferative neoplasms (PN-MPN) are at a higher risk for venous thrombosis. Thromboelastometry may prove efficient to evaluate the patient’s thrombotic risk. In this study, based on data from 114 patients with PN-MPN from a single center in Greece, hemostatic profile was assessed with routine coagulation tests, Rotational Thromboelastometry (ROTEM®), and Platelet Function Analyzer (PFA)-100 and correlated with clinical, laboratory, treatment characteristics, gene mutations and polymorphisms of poly (ADP-ribose) polymerase-1 (PARP-1). According to thromboelastometry parameters, patients with essential thrombocythemia (ET) had a more hypercoagulable status compared to patients with polycythemia vera (PV) and myelofibrosis (MF) while in all patients there was a statistically positive correlation between alpha angle and platelet (PLT) count and between maximum clot firmness (MCF) and PLT count (p < 0.001). In Janus kinase 2 (JAK2) positive patients with PV, the absence of the PARP-1 polymorphism was correlated with thrombosis during follow-up (p = 0.019). Among 47 patients under treatment with aspirin and complete platelet inhibition, no patient had a thrombotic episode in follow-up period (p = 0.006). Based on our results, global hemostatic assays in combination with other parameters could help identify patients with PN-MPN at higher thrombotic risk and adjust the type and dose of appropriate treatment.
Keywords:Philadelphia-Negative Myeloproliferative Neoplasms; Thrombosis; Thromboelastometry; Poly (ADP-ribose) Polymerase-1
Myeloproliferative neoplasms (MPN) are clonal disorders of hematopoietic stem cells characterized by proliferation of one or more myeloid cell lines (granulocytic, erythroid, megakaryocytic). According to the revised 2016 World Health Organization (WHO) classification, PN-MPN include three main disease subgroups: PV, ET, and MF [1]. PN-MPNs can be driven by several genetic mutations, including mutations of the JAK2 gene, the trombopoietin gene (MPL), and the calreticulin gene (CALR). In addition, further mutations in other genes, such as epigenetic modification genes, have been found in PN-MPN. These genes include mainly RNA splicing genes (SF3B1, SRSF2, U2AF1), chromatin histone modification genes (ASXL1, EZH2), and DNA methylation genes (DNMT3A, IDH1, IDH2, TET2) [2,3]. Patients with PN-MPN are at increased risk for arterial and venous thrombosis [4]. Due to the morbidity and mortality of these events, antiplatelet and/or anticoagulant agents are commonly employed as primary and/or secondary prophylaxis [5].
Patients with PN-MPN commonly present with abnormalities in laboratory coagulation tests that are consistent with a hypercoagulable state [6]. Thromboelastometry is a global coagulation assay performed in whole blood that can evaluate the contribution of clotting factors, platelets, and fibrinogen in the clotting process and may prove efficient to evaluate the patient's thrombotic risk [7]. To date, few studies have evaluated the thromboelastometry profile of MPN patients [8-11].
PARP-1 is a nuclear chromatin associated enzyme involved in several important cellular processes, particularly in DNA repair. Εven though rs1136410:C>T or V762A polymorphism of PARP-1 is the most studied single nucleotide polymorphism (SNP) in the PARP-1 gene and probably involved in human carcinogenesis, results from previous studies concerning associations between PARP-1 polymorphisms and MPN risk are inconclusive [12]. We decided to examine PARP-1 in our study because it constitutes a molecule of great interest for our group, both for its role as a prognostic marker in myelodysplastic syndromes and as a possible therapeutic target as described in publications of our team [13,14], [Kontandreopoulou, et al., in press in Blood Advances]. Taking into consideration a numerous of studies which have tried to elucidate the potential involvement of the PARP-1 rs1136410 polymorphism in several cancers (gastric, cervical, lung and breast cancers) [12,15,16], our aim was to evaluate the possible role of this base excision repair gene variant in PN-MPN. Moreover, the possible association between this polymorphism and thrombotic risk could stand, taking into account the protective role that was found in coronary artery disease in a Chinese population [17].
The aim of the present study was to examine the hemostatic profile of patients with PN-MPN and correlate it with clinical, laboratory, and treatment characteristics, JAK2, MPL, CALR gene mutations, and the polymorphism rs1136410:C>T (V762A) of PARP-1.
The present study included patients from one University Hospital in Greece, with a confirmed diagnosis of PN-MPN per the 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. A written informed consent was obtained by all patients and the demographic, epidemiologic, clinical, and laboratory characteristics of the patients were recorded. History of thromboembolic events and occurrence during follow-up were also recorded along with the treatment regimens administered for the management of PN-MPN, including antiplatelet and anticoagulation treatment. At the time of enrollment, all patients were assessed with a complete blood count, routine coagulation tests, such as Prothrombin Time (PT), International Normalized Ratio (INR), activated Partial Thromboplastin Time (aPTT), fibrinogen, and D-Dimers, analyzed with the automatic coagulation analyzer Sysmex (Siemens, Berlin, Germany)]. Furthermore, at the time of sample collection all patients were examined with ROTEM® (Munich, Germany), specifically EXTEM as a screening test for the extrinsic hemostasis system, and several parameters such as clotting time (CT), clot formation time (CFT), MCF, alpha angle, lysis index at 30 minutes (LI30) and lysis index at 60 minutes (LI60) were calculated as described in Figure 1 [18].
A platelet function assay was performed with PFA-100 using collagen-epinephrine (COL/EPI) and collagen-adenosine diphosphate (COL/ADP) cartridges. Closure time was defined as prolonged if >150 sec for COL/EPI or >100 sec for COL/ADP. A closure time >300 sec demonstrated optimal platelet inhibition with aspirin and clopidogrel and a value >150 sec a satisfactory platelet inhibition. Finally, all patients were tested for JAK2, MPL and CALR mutations and the PARP-1 rs1136410 polymorphism.
Mutational analysis of JAK2, MPL and CALR genes was performed on peripheral blood DNA. JAK2 and MPL mutations were detected using standard PCR assays (sensitivity>1%) and CALR mutations were detected using a high-resolution melting analysis (HRMA)-PCR assay (sensitivity>2.5%) [19-21]. The results were confirmed by Sanger sequencing analysis.
For the detection of the PARP-1 polymorphism, samples were collected into ethylenediamine tetracetic acid (EDTA) tubes and processed within 4 hours from collection. Genomic DNA was extracted from cells of peripheral blood sample using a commercially available kit according to the manufacturer’s protocol (Purelink Genomic DNA Mini Kit, Invitrogen, CA, USA). Genotyping of rs1136410 polymorphism consisted of a qualitative PCR followed by an allele-specific restriction enzyme digestion. The primers that were used (5’-GCCATTCACTGTGTTGGACCTT-3’ and 5’-TTGACATCGATGGGATCCTT-3’) amplified a 211 base pair (bp) fragment creating an AciI restriction site in the PARP-1-762A allele. The PCR was conducted as follows: 94 οC for 5 minutes, followed by 30 cycles of 94 oC for 1 minute, 55 oC for 1 minute, 72 οC for 1 minute, and a final extension cycle at 72 oC for 10 minutes. The amplification product was evaluated after electrophoresis in 2% agarose gel stained with ethidium bromide. The amplified DNA was then digested with the AciI restriction enzyme (New England Biolabs, Massachusetts, USA) at 37 οC for 30 minutes. The digestion products were evaluated by polyacrylamide gel electrophoresis in 8% gel (at 85V for 2.5 hours). For homozygous PARP-1-762V patients, one undigested band (211bp) was visualized. In heterozygous patients, three bands (211 bp, 117 bp, 94 bp) were visible, whereas for homozygous PARP-1-762A patients only two bands (117 bp and 94 bp) were seen.
Statistical analysis was performed using IBM SPSS statistics, version 23.0 (IBM Corporation, North Castle, NY, USA). The Mann-Whitney U test and the Kruskal-Wallis test were used to check for differences in continuous variables between two or more than two groups respectively. The Chi-squared test was used for correlations between categorical variables. A p-value under 0.05 was considered statistically significant. Two-sided p-values are reported.
From April 2016 to March 2020, we enrolled consecutively 114 patients (32 with PV, 70 with ET, and 12 with MF) with a median age of 63 years (15-86) as well as 68 healthy subjects to be used as controls only for the SNP/PARP-1 analysis. Their demographic and basic clinical, molecular, and treatment characteristics are shown in Table 1. All participants were followed up until December 2020, with a median follow-up time of 34 months (9-57 months).
Regarding the mutational status, 59 (51.8%) patients were JAK2 positive, 14 (14%) CALR positive, 4 (3.8%) MPL positive, and 37 (32.4%) triple negative for all these mutations.
At the time of sample collection, 69 (60.5%) patients were under treatment with hydroxyurea (HU), 21 (18.6%) with anagrelide, 12 (10,5%) with ruxolitinib, 3 (2.6%) with interferon alpha, and 5 (4.4%) with an alkylating agent. 82/114 patients were treated with antiplatelets [ aspirin 73 (64%), clopidogrel 2 (1.8%), a combination of the two 7 (6.1%)] while 13 patients were under anticoagulant therapy [a vitamin K antagonist 2 (1.8%), a Xa-inhibitor 6 (5.3%), and low-molecular-weight heparin 5 (4.4%)].
At baseline (i.e., before the diagnosis of PN-MPN), 20 (17,5%) patients had a thrombotic episode [6, ischemic stroke; 5, coronary artery disease (CAD); 2, deep vein thrombosis (DVT); 1, carotid stenosis; 1, a combination of ischemic stroke and CAD; 2, pulmonary embolism (PE); 3, visceral thrombosis].
After diagnosis of PN-MPN, 13 (11.4%) patients developed a thrombotic episode, all of them for the first time (3, ischemic stroke; 3, CAD; 4, DVT; 1, a combination of stroke and CAD; 1, a combination of CAD and DVT; 1, visceral thrombosis). Among these, 1 patient in particular developed CAD during the follow up period.
Patients with ET had shorter CFT (median 59 sec, 33-174), compared to PV (median 82 sec, 29-156) and MF (median 101.5 sec, 46-272), p=0.001. Furthermore, MCF was higher in ET (median 69 mm versus 62.5 mm in PV and 53.5 mm in MF), p=0.0001. In ROTEM®, the MCF value is the most commonly used parameter to detect hypercoagulability [22]. Among all patients with high MCF values, only 5/27 (18.5%) had a history of thrombosis before or after diagnosis. Figure 2 depicts a pathologic ROTEM record from a representative patient with hypercoagulable profile.
In all patients, there was a statistically significant positive correlation both between alpha angle and PLT count (rs = 0.474, p <0.001) and between MCF and PLT count (rs= 0.680, p <0.001), (Figure 3 and Figure 4) Moreover, there was a statistically significant strong negative correlation between CFT and PLT count (rs = -0.651, p <0.001). No correlation was found between CT and PLT count (rs = -0.139, p ≤ 0.154).
The same correlations were confirmed when the subgroup of PV or ET patients were examined separately. Especially in PV patients, a statistically significant positive correlation was found between alpha angle and PLT count (rs = 0.550, p =0.002). Moreover, there was a statistically significant strong negative correlation between CFT and PLT count (rs = -0.699, p <0.001) and a strong positive one between MCF and PLT count (rs = 0.676, p <0.001). No correlation was found between CT and PLT count (rs = -0.158, p ≤0.406). In ET patients, there was a statistically significant positive correlation between alpha angle and PLT count (rs = 0.416, p <0.001). A statistically significant strong negative correlation was found between CFT and PLT count (rs = -0.602, p <0.001) and a strong positive one between MCF and PLT count (rs = 0.519, p <0.001). No correlation was found between CT and PLT count (rs = -0.109, p ≤0.391). Finally, in MF patients, no correlations were carried out due to the small number of patients.
A statistically significant correlation of the PARP-1 polymorphism rs1136410 with the presence of thrombosis was found in patients with PV. More particularly, none of the patients with this polymorphism had any thrombotic episodes prior to diagnosis (0/10) while none of the patients with thrombotic episodes prior to diagnosis carry the polymorphism (0/7), p=0.033. No patients with thrombosis during follow-up (4/4) had the polymorphism while none of the patients with the polymorphism had a thrombotic episode during follow-up (10/10), p=0.083. Especially in JAK2 positive patients with PV [19/32 (59.3%)], absence of the PARP-1 polymorphism [11/19 (57.9%)] was correlated with thrombotic episodes during follow-up [4/19 (21%)], p=0.019. The presence of the PARP-1 polymorphism in patients with PV was not correlated with age, sex, fibrosis, splenomegaly, fibrinogen, d-dimers, COL/EPI and COL/ADP, thromboelastometry parameters, hemoglobin (Hb), PLT, white blood cell (WBC) counts, mutation status or treatment with any of the recorded agents. Unlike patients with PV, there was no correlation of thrombotic episodes prior or after treatment with the presence of PARP-1 polymorphisms in patients with ET. More specifically, among 17 patients with the polymorphism, 1 patient had thrombosis under treatment while among 48 patients without the polymorphism 7 had a thrombosis under treatment, (p=0.666). The corresponding values for pretreatment thrombosis are 3/17 and 7/48 respectively, (p=0.713). In JAK2 positive patients with ET (N=32), the presence of a polymorphism did not correlate with a thrombosis at baseline (1 thrombotic episode among 8 patients with the polymorphism versus 4 episodes among 24 patients without, p=1.0) or during follow-up (0 thrombosis among 8 patients with polymorphism versus 4 thrombotic episodes among 24 patient without polymorphism, p=0.55). Among males with ET, 10/25 were positive for the PARP-1 polymorphism while only 7/40 females were positive for the same polymorphism (p=0.047, Pearson Chi-Square). The PARP-1 polymorphism in patients with ET was not correlated with age, fibrosis, splenomegaly, d-dimers, COL/EPI and COL/ADP, thromboelastometry parameters, Hb, PLT, WBC or any of the treatments administered.
Correlations were found between the platelet function parameters and the antiplatelet and anticoagulation therapy as well as with the appearance of thrombotic episodes. Among 80 patients under treatment with aspirin at the time of sample collection (73 patients under only aspirin and 7 under a combination with clopidogrel), 47 patients (58.8%.) had a COL/EPI ≥300 sec while 33 (41.2%) had a COL/EPI <300 sec. Among 9 patients under treatment with clopidogrel (2 patients under only clopidogrel and 7 under a combination with aspirin), only 2 (22.2%) patients had a COL/ADP ≥300 sec while 7 (77.7%) had a COL/ADP <300 sec. COL/EPI was <150 sec in 9/80 (11.25%) patients receiving aspirin and COL/ADP was <150 sec in 4/9 (44.4%) patients receiving clopidogrel. Among those patients with short closure times for COL/EPI and COL/ADP, 1/9 (11.1%) and 1/4 (25%) had a thrombotic episode before diagnosis.
Finally, among 47 patients under treatment with aspirin and complete platelet inhibition (COL/EPI ≥300 sec), no patient had a thrombosis during follow-up (p=0.006). Αfter diagnosis of PN-MPN, 13 thrombotic episodes were noted in patients. Among these patients, 6/13 (46.1%) were under no antiplatelet therapy and 7/13 (53.8%) were receiving aspirin (p=0.701). Among patients under aspirin, 7/7 had COL/EPI <300 sec (median 202.8 sec, 115-295).
None of these patients with a post-diagnosis thrombosis were under anticoagulation therapy at the time of the thrombotic episode (p= 0.529). Nevertheless, none of them had a new thrombotic episode after the initiation of anticoagulants during the follow-up period.
In 101 (88.5%) patients not treated with anticoagulants, the PT, INR, aPTT, fibrinogen, and d-dimer levels did not differ between patients with and without a post-diagnosis thrombotic episode (corresponding level of significance as follows; PT, p=0.479; INR, p= 0.930; aPTT, p=0.187; Fib, p=0.239; d-dimer, p=0.863). Similarly, CT (p= 0.078), CFT (p=0.946), alpha angle (p=0.630), MCF (p=0.673) did not differ in patients with and without post-diagnosis thrombotic episodes.
LI60 was lower (median 95.0 %, 64-100) in patients with thrombosis than in patients without thrombosis (median 96.0 %, 89-100), p=0.024.
No correlation was found either between mutational status and thrombosis (p=0.499) or diagnosis (PV, ET, MF) and thrombotic episodes (p=0.499). Thrombosis, in particular, was not correlated with JAK2 mutation (p=0.506), CALR mutation (p=0.719), or MPL mutation (p=0.878).
Finally, the type of PN-MPN cytoreductive treatment was not correlated with the occurrence of a thrombotic event during follow-up period [HU (p=0.937), anagrelide (p=0.753), ruxolitinib (p=0.117), interferon alpha (p=0.226) and alkylating agent (p=0.536)].
All the parameters that we have examined such as the PARP-1 polymorphism, were tested in correlation with all the clinical and laboratory parameters (age, gender, diagnosis, mutation status, complete blood count, routine coagulation tests, ROTEM®, PFA-100, history of thrombotic episodes, cytoreductive therapy, anticoagulation and antiplatelet treatment) as mentioned above in detail. Multifactorial analysis was not conducted because of the absence of well-established prognostic factors of thrombosis and due to the fact that the majority of the patients were under treatment.
The pathogenesis of thrombosis in PN-MPN patients is multifactorial and not well understood yet. Pathophysiological mechanisms probably involve hemodynamic changes, such as increased viscosity, increased levels of microparticles (MPs) with procoagulant activity, and increased cytokine expression leading to a general inflammatory response. Abnormalities of all MPN-clone-derived blood cells also play a role. More specifically, activated PLTs express P-selectin and tissue factor, release MPs, and provide a catalytic surface for the generation of thrombin. Biochemical changes in the cell membrane and content of red blood cells (RBCs) may lead to aggregation of RBCs and impaired blood flow. In addition, activated neutrophils release proteolytic enzymes and reactive oxygen species and can activate or damage PLTs and endothelial cells as well as impair some coagulation proteins [23-25].
Regarding therapy in PN-MPN, low-dose aspirin 75 to 100 mg once daily for thrombosis prevention is a standard of care in low-risk patients with PV and ET [26,27]. Cytoreductive therapy is reserved for high-risk patients in both ET and PV and low-risk PV patients who suffer from uncontrolled symptoms, symptomatic splenomegaly, and intolerance to phlebotomy [28,29].
Anticoagulation therapy, such as vitamin K antagonists, direct oral anticoagulants, and low-molecular-weight heparin, is recommended for arterial and venous thrombotic episodes and long-term anticoagulation to prevent recurrent thrombosis should be taken into account according to the kind of the episode [30].
A number of studies have provided evidence that the PARP-1 rs1136410 polymorphism may be involved in cancer development, such as gastric, cervical, and lung cancers, among Asians [12,15], but the same polymorphism seemed to reduce the breast cancer risk and delay progression in a Chinese population [16]. Moreover, decreased risk of cancer was found among Caucasians with this polymorphism, particularly of glioma [12,15]. On the other hand, no association has been found between the PARP-1 rs1136410 polymorphism and individual susceptibility for PN-MPN in a Portuguese population [31]. Our study in Caucasians, showed that the presence of this polymorphism in patients with PV was correlated with an absence of thrombosis prior to diagnosis whereas the absence of this polymorphism in JAK2 positive patients with PV was correlated with thrombotic episodes after diagnosis. To our knowledge, this is the first report of such a finding in PV patients. On the other hand, there are few studies correlating PARP-1 to the presence of thrombosis in the general population. The PARP-1 rs2271347 polymorphism was significantly associated with increased ischemic stroke risk in a Chinese population [32] whereas greater PARP activity in circulating leukocytes was found to have a possible causal role in the development of obstructive coronary artery disease among Chinese patients with diabetes mellitus [33].
Global assays, such as thromboelastometry, may be more useful than conventional hemostatic laboratory tests in depicting the hypercoagulable state in oncologic and hematologic diseases [34]. Thorson et al. demonstrated that intra-abdominal malignancies are associated with hypercoagulable ROTEM parameters including decreased CFT, increased alpha angle and increased MCF [35] while Davies et al. noted that, compared to controls, patients with lung cancer have reduced CT, increased MCF and increased alpha angle [36]. In our study, patients with ET were found to have a hypercoagulable profile, characterized by higher MCF and shorter CFT values compared to patients with PV and MF, a finding similar to previous studies. More specifically, Tripodi et al. noted a procoagulant imbalance in ET and MF patients with shorter CFT and higher MCF when compared to controls while the same pattern was not found in PV patients [9]. The hypercoagulable state notably in ET according to ROTEM parameters, was also confirmed in other studied groups with ET patients compared to healthy controls based on CFT and MCF values [10,11]. PN-MPN are characterized by thrombotic complications and ROTEM® could be used in those patients to predict thrombotic risk according to parameters such as MCF [10]. However, in our study only 5 patients with high MCF value experienced a thrombosis in the past. Larger prospective studies would be needed to clarify if ROTEM parameters could predict hemostatic balance and thrombotic risk in PN-MPN patients.
In our study, according to LI60 values, patients with a thrombosis had a slightly bigger hyperfibrinolytic status compared to those without a thrombosis.
Moreover, according to our data, significant correlation was found between certain ROTEM® parameters and platelet counts. In particular, patients with PV and ET with high MCF and large alpha angle had high platelets while patients with high CFT values had low platelets. Similar observations have already been published not only in patients with PN-MPN [8,11] but also in patients with solid tumors [22].
PFA-100 may be useful for the monitoring of treatment with antiplatelet agents not only in the general population but also in MPN patients [37,38]. Moreover, in a large single-center study on a Korean general population including patients with malignancies, among patients receiving only clopidogrel a higher frequency of malignancies was found in the group with normal values of COL/ADP than in the group with prolonged values [39].
In our study, there was evidence that the antiplatelet therapy may not be sufficient in a significant percentage of patients with PN-MPN as 11.25% of patients under treatment with aspirin had COL/EPI <150 sec and 44.4% of patients under treatment with clopidogrel had COL/ADP < 150 sec. Furthermore, the PFA-100 assay could be used to identify patients at higher risk for thrombosis except from identifying patients who are resistant and don’t respond to antiplatelet therapy. Indeed, shorter COL/EPI values as well as shorter COL/ADP values have been linked to higher risk for thrombosis not only in PN-MPN patients but also in patients with acute myocardial infarction [40,41,42]. However, our small sample size could not confirm such finding. Larger studies are needed to fully evaluate these findings and their clinical relevance.
Unlike the findings of other studies where JAK2 mutation especially in ET patients increases the risk of thrombosis [43,44], our data showed that mutational status does not affect the hypercoagulable state of these patients. In addition, this study showed that the type of cytoreductive therapy for PN-MPN patients does not affect their thrombotic profile.
The limitations of the present study are the small number of patients with MF mainly due to the fact that the enrollment of the patients was consecutive and not selective and the fact that hemostatic parameters were assessed only at one time point (the time of the enrollment) and only in MPN patients since a control group was not included.
Based on the results of the present study, we would like to suggest the following for patients with PN-MPN. All PN-MPN patients regardless of their risk group should receive antiplatelet therapy with aspirin and should be considered to have a platelet function assessment with PFA-100. If COL/EPI is especially <150 sec, one could consider the increase of aspirin dose from once to twice daily in order to achieve a better antiplatelet response as showed also by Rocca et al in a randomized multicenter double-blind trial in patients with ET [45,46]. Especially JAK2 positive patients with PV could be assessed for the detection of the PARP-1 polymorphism rs1136410 because the absence of this polymorphism may be a prognostic factor for the development of thrombotic episodes. According to our data that no patient under anticoagulant therapy had a new thrombosis despite the small number of them (only 13/114), we recommend that all high-risk PN-MPN patients under anticoagulants due to a thrombosis should be thought to continue this therapy in addition to cytoreductive therapy throughout the course of their disease due to an increased risk for recurrence of thrombotic episodes especially those with splachnic vein thrombosis [30,47,48].
The presence of the PARP-1 polymorphism rs1136410 in patients with PV and especially in JAK2 positive ones and its correlation with thrombotic episodes prior to diagnosis or/and after the initiation of therapy should be approached in further larger studies of PN-MPN patients. Global hemostatic assays could be incorporated in predictive scores along with other hematological parameters and mutational status in order to identify patients with PN-MPN at higher risk for thrombosis and to guide clinicians for the type and dose of appropriate treatment (cytoreductive and/or anticoagulants).
N.G. analyzed the results, drafted the manuscript, contributed in the conceptualization and methodology; P.D. analyzed the results and contributed in the conceptualization and review of the final manuscript; M.P. contributed in the conceptualization and methodology and reviewed the final manuscript; C.N.K., A.G., D.K., T.K. and K.Z. performed the experiments; S.C., M.D. and M.M. provided clinical samples and reviewed the manuscript; G.K. contributed in the analysis and validation of the results; N.A.V. contributed in the conceptualization and methodology, reviewed and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
The study was approved by the Institutional Review Board.
Informed consent was obtained from all subjects involved in the study.
Data is available upon reasonable request.
We would like to thank Ms. Evita C. Alexopoulos for copy-editing the final manuscript.
The authors declare no conflict of interest.
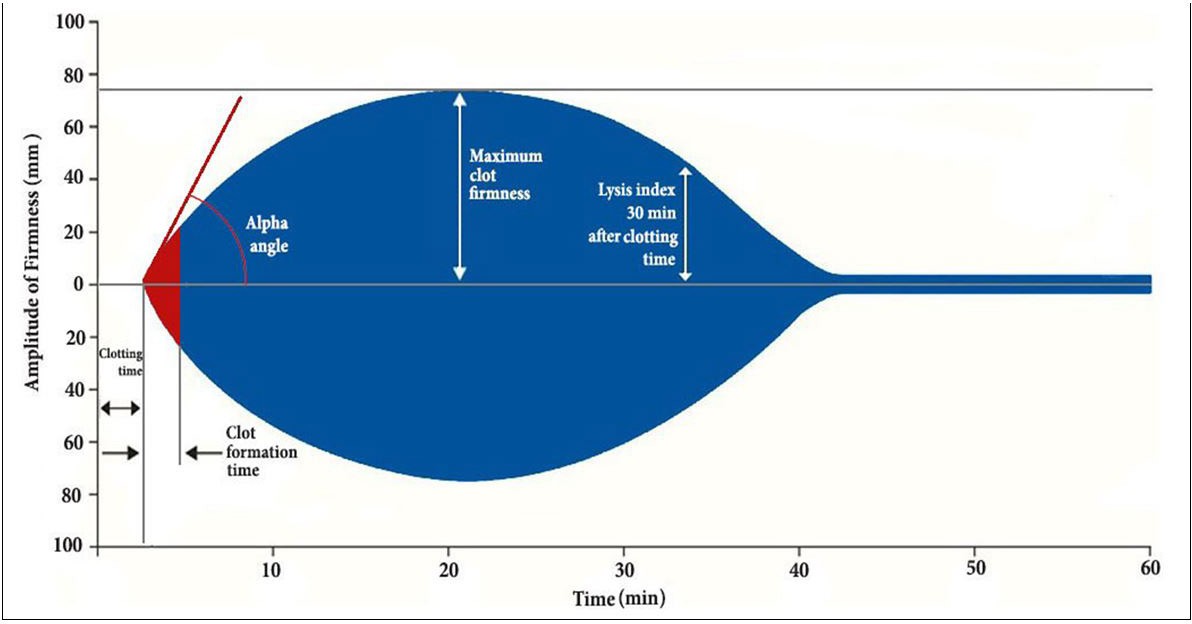 |
| Figure 1: ROTEM� parameters
Clotting Time (CT): corresponds to the time from the beginning of the analysis until the start of clot formation. Shortening of CT indicates hypercoagulability (reference values 38-79 sec); Clot Formation time (CFT): the time from the initiation of clotting until a clot firmness of 20 mm is detected. Shortening of CFT indicates hypercoagulability (34-159 sec); maximum clot firmness (MCF): represents clot firmness and overall clot stability. A low MCF represents a weak clot with severe bleeding risk (50-72 mm); alpha angle: the angle of tangent between 0 mm and the curve when the clot firmness is 20 mm. The more acute it is the more hypocoagulable the patient�s state; Lysis Index at 30 and 60 minutes (LI30), (LI60): the percentage of remaining clot stability in relation to the MCF value at 30 and 60 minutes after CT respectively. A low LI value indicates hyperfibrinolysis (reference values > 15%) |
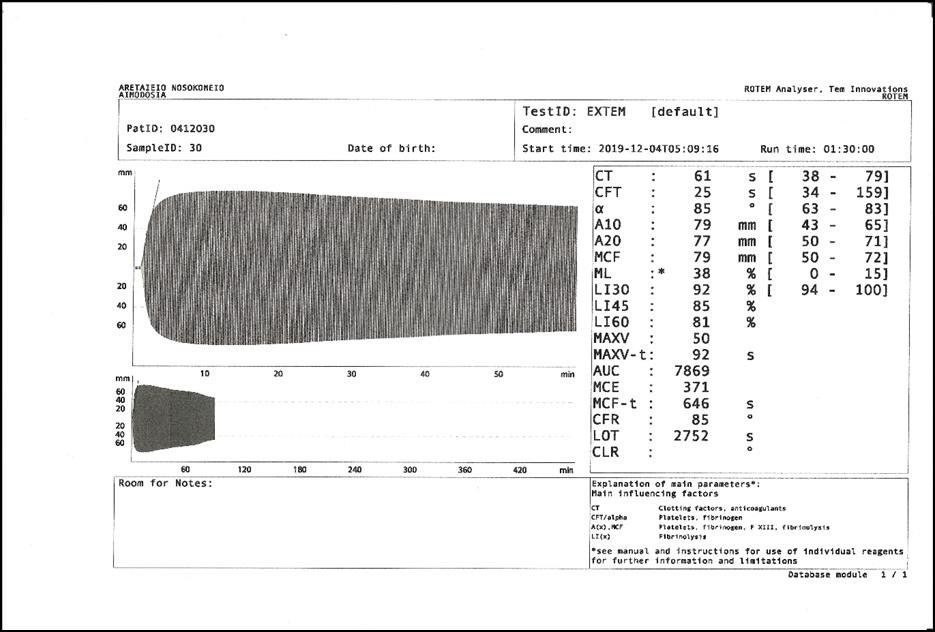 |
| Figure 2: Pathologic ROTEM� record of a 70 years old woman with ET under aspirin and a history of DVT before diagnosis |
 |
| Figure 3: The correlation between platelet count and alpha angle EXTEM (rs = 0.474, p < 0.001) |
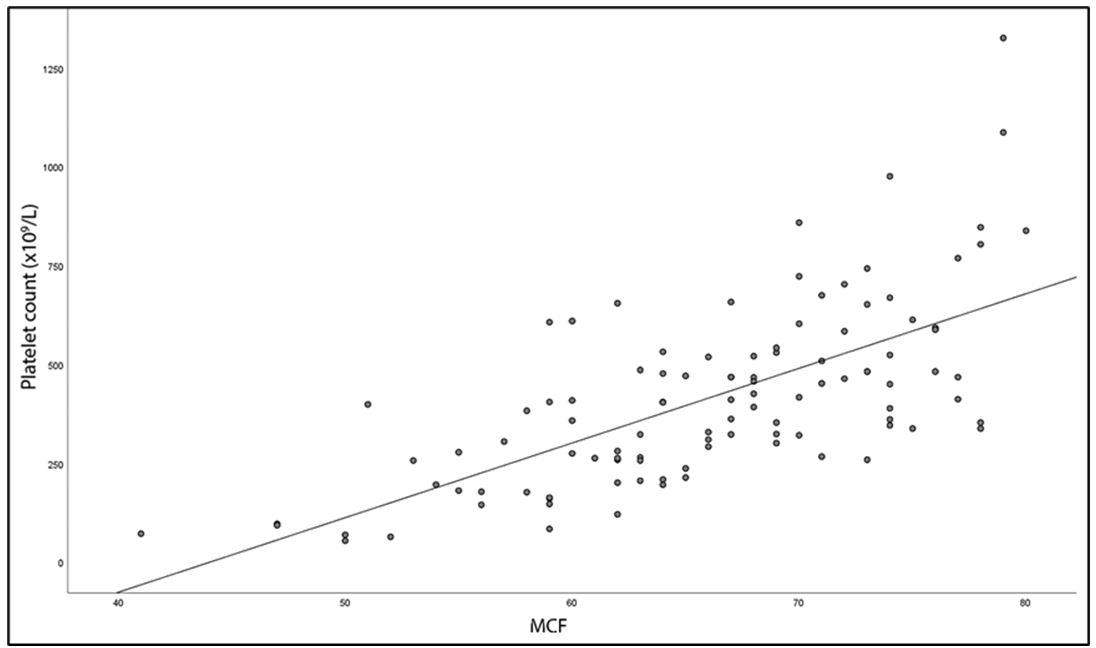 |
| Figure 4: The correlation between platelet count and MCF EXTEM (rs = 0.680, p < 0.001) |
Characteristics |
Result |
Number of patients, N (%) |
114 (100) |
Sex (Male), N (%) |
58 (50.8) |
Age (years), median (range) |
63 (15-86) |
Diagnosis, N (%) |
114 (100) |
PV |
32 (28) |
ET |
70 (61.4) |
MF |
12 (10.5) |
Antiplatelet therapy, N (%) |
82 (71.9) |
Anticoagulant therapy, N (%) |
13 (11.4) |
Thrombosis before diagnosis, N (%) |
20 (17.5) |
Thrombosis after diagnosis, N (%) |
13 (11.4) |
Mutation status, N (%) |
|
JAK2 |
59 (51.8) |
CALR |
14 (14) |
MPL |
4 (3.8) |
Triple negative |
37 (32.4) |
PARP-1 polymorphism, N (%) |
28 (26.7) |
Absent |
77 (73.3) |
Heterozygous |
26 (24.8) |
Homozygous |
2 (1.9) |
Table 1: Patients� demographic, clinical, therapy, and molecular characteristics at the time of sample collection






































