Top Links
Journal of Gastroenterology and its Complications
ISSN: 2575-5501
Reasons for hospitalization of HIV-positive patients in Riga East clinical University hospital, Latvia, 2014
Copyright: © 2016 Seikals K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
HIV/AIDS occupy a special place among other diseases. It is Incurable and frightening disease, causing anger and denial. In the consciousness of people it is associated with violations of the norms of morality and causes condemnation. Those affected by it, have to face with a unique attitude of society - different than for the rest of the incurable diseases: blaming, abuse, discrimination, avoidance. It is important to overcome this barrier of fear, ignorance and prejudice. Knowledge about this deadly infection is the most important part of prevention. HIV/AIDS can affect anyone, regardless of his social status, ethnicity and financial position. The more professional information about HIV infection will be available, the less people will be unwarranted by the sense of fear for their own and relatives’ safety [1].
In Latvia cases of HIV/AIDS have been registered since 1987. The first instances of HIV in Latvia, like elsewhere in the world, were discovered among men who had it through homosexual contact. Until the mid-nineties, the infection spread practically through the way of sexual contact, and every year relatively few cases of HIV were discovered. A new milestone was marked in 1997, when HIV infection started to spread between intravenous drug users (IDU), and due to the use of shared drug injecting equipment, spread very quickly in this population [2].
According to the data of Infectology Centre of Latvia, in Latvia in the 1st October 2014, there were registered 6117 HIV-infected patients, but the actual number of infected people are two to three times higher, as most of them do not even suspect that they are infected [3].
In the year two-thousand-and-thirteenth, there were registered 340 new HIV cases. The actual number of people infected with HIV in Latvia is estimated one and a half to two times higher. Number of registered patient’s according to transmission was vertical transmission (mother- child) -10, heterosexual contacts - 125 cases. In its turn, 27 HIV cases were detected after homosexual contacts, while after drug injection – 77.
In 102 cases – route of infection was unknown [2]. In 2013, more than 35 million people live with HIV, of whom 3.2 million were aged less than 15 years. About 2.1 million were diagnosed, of which 240 thousand were aged less than 15 years. In the year two-thousand-and-thirteen 1.5 million people died of AIDS. Only 12.9 million people (37% of the total number), who have been diagnosed with HIV have access to antiretroviral therapy. On average in 2013 in Eastern Europe and Central Asia, 110 000 new cases of HIV infection were registered. Increasing the number to 1.1 millions of HIV-infected in these regions [4,5]. From the total number of registered patients, 4387 patients are in the HIV/AIDS registration department of Infectology Center of Latvia. Of which 1461 patients already have AIDS. Every year 200 - 230 new cases are registered [3].
Currently, 1011 patients receive treatment therapy. It is 100% compensated by the state, but it should be noted that Latvia is the only country in the European Union, where the treatment is started from 200 cells. For the state treatment of one patient costs from 350 euro’s up to 7000-8000 euro’s per month [3].
The 14th European AIDS Conference that was held in 2013 emphasizes specific problems that have to be faced in Eastern Europe. Those are, that part of the patients is infected with co-infections tuberculosis and Hepatitis C, which made implementation of treatment program very difficult. A big problem is the high incidence of intravenous drug use as well. It is estimated that morbidity has increased for 140% over the past decade. Besides, Eastern Europe is the only region in the world, where number of AIDS-related deaths continues to grow [6]. In 2011 Latvia was in the second place by prevalence of HIV/AIDS patients, Estonia took the first place [7]. Studies show that between health care workers there is often negative perception about people with HIV. Besides, preconception against HIV infection is much higher in comparison to other diseases. It has been proven that negative attitude towards HIV patients worsens their treatment. Research has shown that, although the majority of health care workers understand how the HIV is transmitted, they still admit fear of the disease, and those who are the most afraid, in great extent maintain and foster a stigmatizing view [8].
In result of the research to obtain information and analyze the current situation and pending matters about the reasons for the hospitalization of HIV-positive patients in “Infectologycentre of Latvia” during the period from 1st January 2014 until 31st December 2014.
1. Assess causes and reasons of HIV hospitalization, and compare with the literature data.
2. Evaluate current problems associated with treatment, medical care of patients and causes of hospitalization.
HIV is an abbreviation from English Language of Human immunodeficiency virus, in Latvian it is-cilvekaimundeficitaviruss (human immunodeficiency virus). HIV infection is a condition, when HIV is located in the human body and as a result, between micro- and macro-organisms a variety of mutual relationships are formed, which may result in deviations from the norm in various laboratory and clinically detectable endpoints, but it is also possible that any deviations cannot be detected for a long period of time [9,10].
HIV-1 and HIV-2 are retroviruses that belong to the family of Retroviridae, subfamily of Lentivirus. They are containing capsids, diploids, single-stranded RNA positive viruses, which with intermediate DNA that is integrated in the carrier cell DNA, are capable to replicate within host organism [11].
Source of infection is HIV-infected people, in whose blood is circulating the virus. It is possible to become infected after exposure with infected body fluids, sexual contact, iatrogenic, intravenous injections, during vaginal childbirth, as well as breast-feeding, especially if nipples are inflamed and/or chapped [12]. HIV causes depletion of cellular immunity, characterized by reduction of helper T-cells or (CD4) cells. Reduction of CD4 cells leads to illness with opportunistic infections or neoplastic processes [13]. Highest risk of becoming infected is from human suffering with an acute HIV infection in the period of seroconversion, because in this phase viral load is very high [10].
Once HIV enters the body, it is able to infect a variety of differentiated cell types. The most important is the CD4 lymphocytes or helper cells and monocytes, macrophages, as well as follicular dendritic cells of the lymph modes, brain astrocytic, gut epithelial and vascular endothelial cells, as well as many other cells [14].
With animal studies it was proved that Langerhans cells are the first to be infected. It later connects with the CD4 lymphocytes and spreads deeper in tissues. In humans primary viremia of plasma and dissemination of the virus appears in 4-11 days after the virus get through mucous membranes [13].
When the genome of HIV virus gets into the cytoplasm, the information of the RNA virus is transferred to DNA with the help of reverse transcriptase. The virus transforms into provirus of DNA, and can be found in the body for a long time in a condition known as “carrying”. Thereby it follows that most important in the pathogenesis is activation of provirus [10]. At this stage, the infection has established proviral reservoir. This reservoir consists of persistently infected cells, which are generally macrophages that continuously release the virus. It provides both formation of new reserves and maintains an active infection [15]. Activity of virus replication positively correlates with clinical manifestations. At the stage when there are not clinical manifestations, the amount of damaged CD4 cells decreases about thousand times and even more, because the virus load reduces as well, i.e. the amount of virus [16]. Proviral reservoir correlates directly with constant virus load and inversely correlates with anti-HIV CD8 T-cell response. Early invasive treatment of acute infections can lower pro viral load, but overall, treatment of newly infected patients that are after conversion, does not provide long-term benefits. Proviral reservoir is measured by DNA polymerase chain reaction (PCR), which seems to be stable indicator. Though, it decreases by using invasive anti-virus therapy [13]. As the main target of HIV are CD4 cells, then after their gradual degradation, the ratio of CD4 and CD8 tilts in favor of the latter [10].
Possibility to restore the immune system is reduced, if treatment is initiated too late, or if after the initiation of treatment the number of CD4 T-cells falls below 200 c. /ìL and below [13].
HIV-related illnesses: Incubation period of HIV infection lasts from two weeks to three months. Incubation period ends either by a clinical manifestation known as acute retroviral syndrome, or with formation of antibodies against HIV [10]. Clinically HIV infection is divided into three phases: acute seroconversion that can manifest itself as acute HIV syndrome, asymptomatic infection during which HIV latency can be seen and AIDS during which opportunistic infections become apparent (Figure 1) [13].
Acute retroviral syndrome is characterized by non-specificity, i.e., there are no specific symptom clusters that would help to identify infection with certainty. However, identifiable symptoms are fairly typical, and sometimes recall infectious mononucleosis, sometimes rubella, sometimes ARIs (acute respiratory infection), etc [10]. Approximately 40-90% of HIV-infected will have acute symptoms initially. Seroconversion can last for 3 - 12 weeks, up to 6 -12 months after infection. Manifestation of HIV infection is usually characterized by fever 96%, lymphadenopathy 74%, pharyngitis 70%, maculopapular rash 70%, myalgia 54%, headache 32%, diarrhea 32%, nausea and vomiting 27%, hepatosplenomegaly 14%, weight loss 13%, neurological symptoms 12%, oral candidiasis 12%. On average symptoms last one to three weeks [15,16].
Latent stage or process can be observed in cases, when the human body is able to maintain the immune system in such condition, that the opportunistic diseases do not accede. Usually, this stage lasts for five to seven years and begins after primary or acute expression of phase, and appearance of antibody against HIV. Symptoms of latent phase do not become apparently clear. The most characteristic symptom is generalized lymphadenopathy, which is characterized by appearance of asymmetric lymph modes, the diameter of which is 1 - 2 centimeters. While palping they are flexible and are not related with surrounding tissue. Such physical lymph mode does not change for about three months. This is called a generalized lymphadenopathy. It is considered that the latent stage duration positively correlates with CD4 lymphocyte quantity, but their reduction below 350 generally coincides with the HIV infection and its related clinical pathology manifestations, indicator diseases accede if the CD4 cell count is 200 or less, it extremely deteriorates patient’s health [13].
Secondary pathology or disease stage is characterized by a bacterial, viral, fungal, protozoa and/ or oncologic, as well as with manifestation of other diseases on the background of weakened immune system. In fact, there is a transition to AIDS or AIDS associated complex, which in its turn is clinically characterized by physical and mental working capacity, night sweats and periodic increases in body temperature, unstable gastric emptying and weight loss for about 10% within a few months [10].
HIV opportunistic diseases: When the immune system is sufficiently weakened, and significant opportunistic infections begin to develop, person is considered to be AIDS patient. For surveillance purposes, if the CD4 T-cell count is less than 200 c. /μL, then AIDS is diagnosed, although some opportunistic infections begin to develop when the CD4 T-cell count is more than 200 c. /μL, as well as, some patients with CD4 counts below 200 c. /μL may remain relatively healthy. Many opportunistic infections and disease conditions are used to note when the HIV infection has progressed to AIDS. The overall incidence and conditions of these infections may occur both rarely and commonly, but all of them are rarely found in immunocompetent persons (Table 1) [17].
AIDS indicator diseases are pathologies of a non-infectious nature and/or infectious diseases, which are the most common for HIV-infected patients with immunodeficiency. Opportunistic diseases are stimulated by infectious agents that most frequently join HIV-infected patients with immunodeficiency and make the patient’s condition more severe [10].
Reduction of T-helper (CD4) cell count causes changes in relation of CD4/CD8 T-cell and causes deregulation in production of B-cell antibodies. The immune response to certain antigens starts to weaken, thus the HIV patient is unable to respond adequately to opportunistic infections. Because of the defect in cellular immunity, infections tend to be of non-bacterial nature (fungi, virus).See correlation between HIV infection complications and CD4 cells count in Table 2 [13].
Opportunistic infections reflect the most common pathogens that are widespread in this area. For example, people with AIDS in the USA are infected more often by Pneumocystis and Candida species, homosexual men are more likely to become ill with Kaposi’s sarcoma, because there is high level of co-infection with HHV-8 (human herpesvisrus-8), tuberculosis is common in lesser developed countries [13].
Bacterial pneumonia occurs 25 times more often among HIV-infected patients than in the general population, incidence of which increases as soon as the number of CD4 cell count decrease [18]. Between HIV-infected patients with bacteraemic pneumococcal pneumonia 14-day mortality is higher than for HIV-negative patients [19]. In a retrospective studies in France, in which HIV-positive patients with respiratory problems were examined before HAART (highly active antiretroviral therapy) and HAART era, it was found that opportunistic diseases such as pneumococcal pneumonia and chronic bronchial disease exacerbations are associated with reduction of Gram-negative bacteria [20].
Increasing failure of the immune system or its insufficiency within five years, leads to development of widespread AIDS stage. This stage is characterized by two main directions of clinical manifestations, namely generalization of opportunistic diseases and disseminated neoplasia. Any pathogens but sometimes also conditionally pathogenic microorganisms are capable to cause extremely severe clinical conditions for patients. Currently, 26 diseases are recognized as AIDS indicator diseases, but this number may vary [10,14].
These diseases are included in category C of HIV infection; a further breakdown of HIV patients after clinical conditions and CD4 cell count has to be viewed. Even after the initiation of therapy, and effective suppression of virus load, patients with persistently low CD4 counts are still in high risk of opportunistic infections. In general, all patients still have a relatively high risk of opportunistic infections and other AIDS associated conditions in the first six months of the antiretroviral therapy use [21]. It is important to remember that the secondary pathology manifestations are possible during prolonged remission for several months or even years. For the assessment of the immunological aspect CD4 cell counts are mainly used [10].
First-line HAART regimen it is recommended that two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) be combined in the first-line HAART regimen. Triple NRTI-based first-line regimens such as ZDV+3TC+ABC and ZDV+3TC+TDF can be recommended in specific circumstances where NNRTI is contraindicated or too complex to manage and have the advantage that they still preserve the PI class for second-line ART. These regimens can be used in the following circumstances: intolerance or resistance to NNRTIs, psychiatric disorders, pre-existing liver disease – an increase of the ALT level by more than 3–5 fold–and established cirrhosis, co-infection with HBV or HCV, HIV-2, infection due to intrinsic resistance to NNRTI class, and co-treatment of TB in women of child-bearing age and where adequate contraception cannot be guaranteed, and when NVP and boosted PIs cannot be used [22].
In resource-limited settings the decision to initiate ART in adults and adolescents relies on clinical and immunological assessment [23]. In order to facilitate the rapid scale-up of ART programmes with a view to achieving universal access to this therapy, WHO emphasizes the importance of using clinical parameters in deciding when to initiate it.
However, it is recognized that the value of clinical staging in deciding when to initiate and monitor ART is improved by additional information on baseline and subsequent (longitudinal) CD4 cell counts. While WHO continues to advocate wider availability of affordable point-of-care CD4 cell count testing, the lack of a CD4 count should not delay the initiation of ART if the patient in question is clinically eligible. WHO encourages national programmes to increase access to CD4 measurement technologies.
The optimum time to commence ART is before patients become unwell or present with their first opportunistic infection. Immunological monitoring (CD4 testing) is the ideal way to approach this situation. A baseline CD4 cell count not only guides the decision on when to initiate ART but is also essential if CD4 counts are to be used to monitor ART (Table 3).
Until recently, BHIVA recommended that individuals with chronic HIV infection should start ART before the CD4 count fell to below 350 cells/ìL. This recommendation was based on evidence from cohort studies that demonstrated and increased risk of disease progression in individuals who delayed ART until their CD4 count was below 350 cells/ìL and the absence of robust evidence from RCTs in which the intervention, comparator and populations were similar to our own setting [24]. Low percentages (0–23%) of co-infected patients have access to hepatitis C treatment [25]. There may be several reasons for this: the efficacy of PEG-IFN and RBV in treating coinfected patients was only published in 2004, and these drugs are not widely available. A great number of patients who continue active drug use do not have access to substitution treatment and/or ART [26].
Many countries lack guidelines for diagnosis and treatment. Evaluation of the severity of HCV disease and treatment requires high technology and skills. Neuropsychological side-effects and toxicity are frequent during HCV treatment. Treatment is very costly [22]. Several studies have demonstrated that patients co-infected with HCV and HIV have more rapid fibrosis progression than mono infected patients, even after taking into account age, sex and alcohol consumption [27,28]. People with HCV/HIV co-infection may have quantitative and/or qualitative deficiency in their immune responses to HCV. HIV accelerates the course of HCV-associated liver disease, particularly in patients who are more severely immune deficient, by increasing: the HCV viraemia level from two-to eightfold, resulting in a significant decrease in spontaneous recovery from acute hepatitis, the risk of mother-to- child and sexual transmission (from averages of 6% to 20% and from 0% to 3%, respectively), and rates of liver fibrosis (two- to fivefold), cirrhosis, decompensation, hepatocellular carcinoma (HCC) and liver- related mortality [27,28].
Side-effects are common with ARVs, especially PIs. LPV/r and NFV can cause severe diarrhoea. LPV/r is associated with hyper lipidaemia (especially high triglycerides). Problems with lipid metabolism can occur with nearly all PIs. Long-term studies of side- effects and increased risk for cardiovascular complications are needed. Toxicity might be a reason for substitution of prescribed ARV to another ARV drug within the same regimen. Switching to another treatment regimen due to toxicity is not recommended [22].
Study material: In a retrospective study were included 167 patients that were hospitalized in Ltd. “Riga East Clinical University Hospital” stationary “Infectology Centre of Latvia”, in the period from 1st January 2014 till 31st December 2014. Randomized data was obtained about patient’s gender, age, HIV transmission, infection duration, employment, CD4 cell count, hospitalization diagnosis, stage of HIV, whether patients are receiving treatment, and whether there is HCV co-infection. Case records were selected with a diagnosis of HIV, and with hospitalization time in the period from 1st January 2014 until 31st December 2014. In the study will be included patients who meet the criteria. Switching criterion – patients with HIV diagnosis that were hospitalized from the 1st January 2014 until 31st December 2014. Exclusion criterion: patients with HIV diagnosis that were not hospitalized in the period from 1st January 2014 until 31st December 2014.
The study will be carried out in accordance with provisions of the Declaration of Helsinki and the Convention on Human Rights. Confirmation for conduct of this study and access to the archives was obtained from Ltd. “Riga East Clinical University Hospital” stationary “Infectology Centre of Latvia”. Study application was reviewed and accepted in Science department of Ltd. “Riga East Clinical University Hospital”; authorization for the conduct of the study was obtained from Ethics Committee of “Riga Stradins University”.
The author wrote study data in the computer program MS Excel, converting (USA) company’s PASW software SPSS version 20.0, for further data processing. Data were expressed in average values ± standard deviation (SD), for the coherence and reliability of data the Chi-square (χ2) test and the Mann-Whitney U test were used. Correspondence of statistical hypothesis level p<0.05 was seen on the basis of the corresponding null hypothesis rejection and acceptance of alternative hypotheses.
Selection of inpatient case-record data: From 167 medical records that were studied, it is known that 88 (52.7%) were women, 79 (47.3%) – men (Figure 2).
It is known that youngest of the 167 patients was 21 years old, the oldest 72 years old. The average age 39. 10 years (SD ± 10. 19) (Figure 3). Using the Mann-Whitney U test, appears a statistically believable difference among hospitalized HIV-positive patient’s gender and the age of patients (p=0.001). The average age of women 36, 93 years (SD ± 10.42) and for men 41, 51 years (SD±9.41) (Figure 4).
After evaluation of infections transmission the following data was obtained from 167 hospitalized patients - 62 (37.1%) IDU, 73 (43.7%) unknown, 8 (4.8%) homosexual way, 24 (14.4%) in heterosexual way (Figure 5).
Using the Chi-square (χ2) test, author concludes that there is a statistically credible difference in the frequency of transmissions between the genders (p=0.001). Of all the patients 20.4% were men, who became infected after IDU (intravenous drug use), for 18.6% reason is unknown.
Of all the patients 16.8% were women, who became infected with IDU; unknown reasons were mentioned by 25.1% and in heterosexual way 10.8% (Table 4).
The average CD4 cell count was 295.16 (SD ± 284.25). The smallest value 3, the largest 1700 (Figure 6).
Most common reasons for hospitalization was opportunistic infection 67 (40.1%), the tumor 3 (1.8%), hematologic disease 6 (3.6%), acute retroviral syndrome 19 (11.4%), gastrointestinal and liver pathology 20 (12%), neurological disorder 13 (7.8%) surgical manipulation 7 (4.2%), nephrological disease 5 (3%) pulmonology 18 (10.8%), dermatological complaints 9 (5.4%) (Figure 7).
Allocation of patients following HIV stages: AI 14 (8.4%), AII 28 560 (16.8%), AIII 23 (13.8%), BI 1 (0.6%), BII 3 (1.8%), BIII 9 (5.4%) CI 4 (2.4%) CII 11 (6.6%), CIII 74 (44.3%) (Figure 8).
Of all hospitalized patients 69 (41.3%) receive treatment, 98 (58.7%) do not receive it. Hospitalized HIV patients in 70 cases (42.17%) were co-infected with HCV (Figure 9).
Processing of statistical data and results: Using the Chi-square (χ2) test, the author concludes that there is a statistically believable difference in incidence of HCV co-infection frequency between patients who have a job and those who are unemployed (p=0.003). Patients, who have a job, HCV was found in only 25.9% (constitutes only 12% of all patients); whereas between patients who are unemployed, it was present in 50% (constitutes 36.5% of all patients) (Table 5).
Using the Chi-square (χ2) test, the author concludes that there is a statistically believable difference in incidence of transmission frequency between the patients who have a job and unemployed (p=0.007).
Unemployed in 45.5% had IDU case record (30.5% from the total number of patients), while 52.7% of working patients do not know how they became infected with HIV (17.4% from the total number of patients) (Table 6). As well as, there is statistically credible difference in the frequency of co-infection between transmission modes 4 (p<0.001). Patients, who admitted that they are IDU, in 63.9% cases, are co-infected with HCV (23.5% from the total number of patients) (Table 7).
Of all patients with CD4<350 c. / mm3 only 42.5% receive treatment, 57. 5% do not receive treatment, and of those who do not receive treatment 77% are unemployed (Table 8).
Of these, 51% are also IDU and in 51. 1% of cases are co-infected with HCV (Table 9).
Of all patients with CD4<350 c. / mm3, opportunistic infections were found in 51.9% (Figure 10). Using the Chi-square (χ2) test, the author concludes that there is a statistically credible difference of transmission and working presence in the group of patients with CD4<350 c. / mm3 and opportunistic infections (p=0.017). It shows that majority of patients (36.5% of all patients in this group) consist of unemployed, who had IDU case records (Table 10).
Using the Chi-square (χ2) test, the author concludes that there is a statistically credible difference in the frequency of co-infection incidence among hospitalized HIV-positive patient diagnoses (p=0.005), but not in the cases of opportunistic infections (p=0.470) (Table 11). Among all the cases of leading diagnoses, of which HCV co-infection was expressed, were liver and GIT (gastrointestinal) pathology in 20% (8.4% of all cases) and surgical interventions (liver biopsy) 8.6% (3.6% from all cases) (Table 12).
Using the Chi-square (χ2) test, the author concludes that there is a statistically credible difference in the frequency of hospitalization diagnoses with CD4 levels (p=0.023). Opportunistic infections, with number of CD4 cells <200 c. / mm3 was 59. 7%, but from all patients it constitutes 24% (Table 13).
There was also noticed a small (R≈0.2) correlation between the duration of the infection and CD4 counts (p=0.005). Especially, in the group of patients with CD4<200 c. / mm3 and who do not receive treatment (p=0.03; R≈0.3). As there is a CD4 count of correlation with the frequency in hospitalization of opportunistic infections, it can be concluded, that patients who are suffering from HIV infection for a long time are exposed to a higher risk of being hospitalized, particularly the group of patients with CD4<200 c. /mm3 and those who do not get treatment (Figure 11).
Using the Mann-Whitney U test, a statistically significant difference among hospitalized HIV-positive working patients and duration of infection (p=0.004). The average duration of infection for the working patients during the hospitalization is 5.35 years (SD ± 5.351), for unemployed 7 and 35 years (SD ± 5.699) (Figure 12).
Spread of HIV infection is still a topical issue. Care for patients, HIV-associated illness and treatment for opportunistic diseases continues to represent not only the financial burden for medical care - 350 euro up to 7000-8000 euros, but also creates a need to examine the actual problems associated with reasons for the patient hospitalization. Assess current disease problems biopsychosocial health point of view, the assessment of both the patient’s organic damage and social problems [3]. From the studied 167 medical records, it is known that 88 (52.7%) were women, 79 (47. 3%) men and their average age 39. 10 years (SD ± 695 10. 19), average age of women 36. 93 years (SD ± 10.42) and age of men 41.51 years (SD ± 9. 41).
The most common reasons for hospitalization were opportunistic infection 67 (40.1%), primarily gastrointestinal fungal infection (83%), gastrointestinal and liver pathology 20 (12%), acute antiretroviral syndrome 19 (11.4%), pulmonology 18 (10.8%), neurological disease 13 (7.8%), hematologic disease 6 (3.6%), dermatologic disease 9 (5.4%), surgical manipulation 7 (4.2%), nephrological disorder 5 (3%), the tumor 3 (1.8%). Hospitalization causes are studied in other countries as well, and such data varies in works of authors within a wide range, where as the main reasons are mentioned opportunistic infections (52%-46.5%), surgical treatment (10%), haematological disorders / tumors (8%), cardiovascular disorders (8%), neurological diseases (7%), gastrointestinal and liver abnormalities (6%) [29-31]. Using the Mann-Whitney U and Chi-square (χ2) tests, the author saw a close correlation (p<0.05) that HIV patients who are unemployed and who are co-infected with HCV are hospitalized more common (50%), most of these patients have an anamnestic IDU (58.9%), and 64.3% of these patients do not receive therapy. As well as, most of the patients constitute in the stage CIII 44.3%.
Of all patients with CD4<350 c./ mm3 only 42% receive treatment, 57.5% do not receive treatment, and 77% of those who do not receive treatment are unemployed, of which 51% is also IDU and 51% have HCV co-infection. Of all patients with CD4<350 c. / mm3, opportunistic infections were found in 51.9%. Using the Chi-square (χ2) test, author concludes that there is a statistically believable transmission and difference in existence of employment in the group of patients with CD4<350 c. / mm3 and who have opportunistic infections (p=0.017). It shows that the majority of patients (36.5% of all patients in this group) consist of the unemployed, who are anamnestic IDU.
The author concludes that these unfavorable social conditions related to unemployment and IDU anamnestic, contributes the progression of disease, and such patients are a lot unresponsive in receiving therapy, respectively they are increasing the frequency of HIV patients hospitalization. The other main connection which the author wishes to point out is the late initiation of therapy, it is when a CD4<200 c./ mm3 also predicatively increases the frequency of HIV patients hospitalization.
Thus, increasing both the financial burden of patient care and treatment of opportunistic infections and deteriorating course of patient illness and health.
Overall, the author concludes that it is important to assess patient’s hospitalization from biopsychosocial perspective. It is necessary to reduce unemployment, especially in the risk group of HIV patients, who are IDU because this group is exposed to a higher risk of hospitalization, and more likely to receive irresponsive treatment. To promote preventive measures, vaccination, prophylaxis for opportunistic infections, that would reduce patient’s hospitalization frequency, as well as initiate earlier antiretroviral therapy.
HIV infection is a serious, chronic, manageable disease. Currently it is impossible to initiate treatment if the patient has less than 200 immune system cells per mm3, but situation is such that we spend public funds for the treatment of these patients anyhow, while treating other diseases they would not have, if the treatment would be started from 350 cells, as it is in other European Union countries [3].
1. The most common reasons for hospitalization were opportunistic infection 67 (40.1%), mainly gastrointestinal fungal infection (83%), acute retroviral syndrome 19 (11. 4%), gastrointestinal and liver pathology in 20 (12%) pulmonology 18 (10.8%). Thereby, the author recommends to control and contribute preventive measures of opportunistic infection more rigorously, to immunize patients against HBV (hepatitis B) and S. pneumoniae, to treat hepatitis C infection, regularly perform annual physical examination by family physician.
2. From all patients with CD4<350 c./mm3 opportunistic infections were found in 51.9%, the majority of which (36.5% of all patients in this group) are unemployed, who are anamnestic IDU (injection drug users). Statistically reliable data show that there is connection that patients, who are at high social risk – unemployment, IDU anamnestic, refuses and are not responsible for therapy, and more often remain in stationery due to the opportunistic infections. Therefore, the author recommends promotion of social rehabilitation for this risk group of patients, to promote their integration into society and to reduce stigma in society and among patients.
3. The author concludes, that it is statistically credible (p=0.023), that amount of opportunistic infections increases 59.7% (24% of all 793 patients) when the number of CD4 cells is <200 c. / mm3. Author recommends to start antiretroviral therapy at early stage, as it is recommended in the latest guidelines - to start antiretroviral therapy when CD4<350 c. / mm3.
4. There was also noticed small (R≈0.2) correlation between the duration of the infection and levels of CD4 (p=0.005), especially in the group of patients whose CD4<200 c. / mm3 and who undergo treatment. As there is a correlation with level of CD4 and with hospitalizations incidence of opportunistic infections and duration of infection; the author concludes that patients who are suffering with HIV infection for longtime and are irresponsible for the therapy are at higher risk of being hospitalized. Hence, there is necessity for careful and timely risk assessments of health problems by controlling the CD4 levels, as well as education of patients and medical staff about options of treatment and care taking of HIV patients.
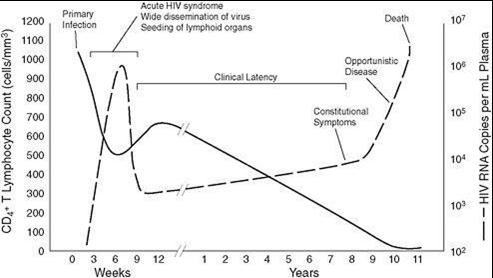 |
Figure 1: Progression of untreated HIV infection in relation to the CD4 cell count and virus load |
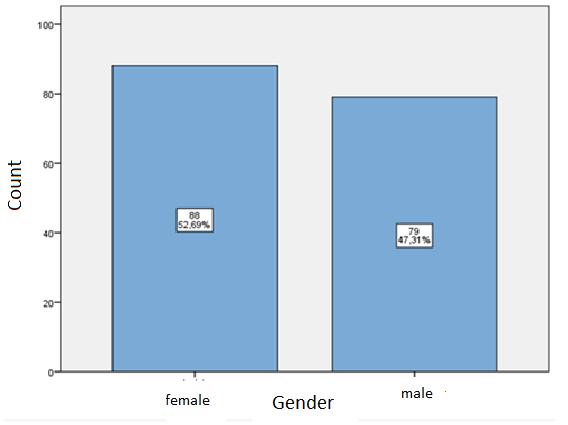 |
Figure 2: Distribution of patients by gender |
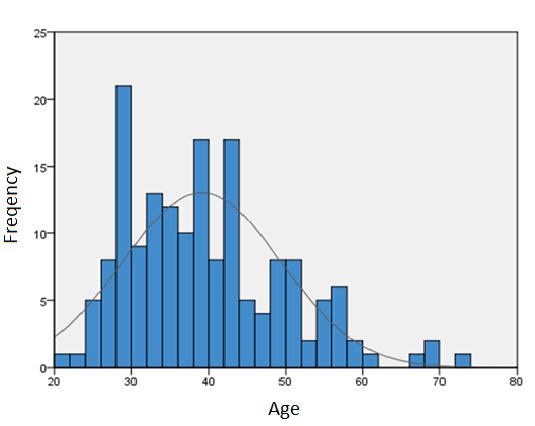 |
Figure 3: Patient age histogram |
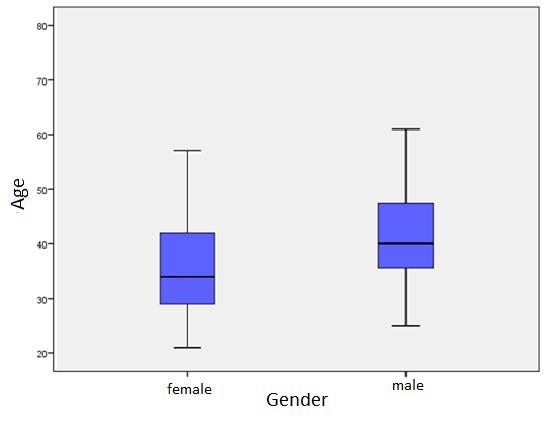 |
Figure 4: Patient distribution by gender mean ages |
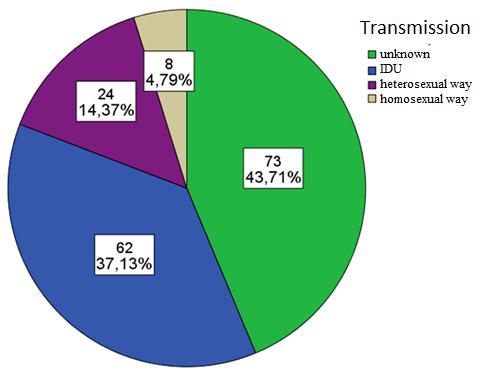 |
Figure 5: Proportion of patients of HIV transmission |
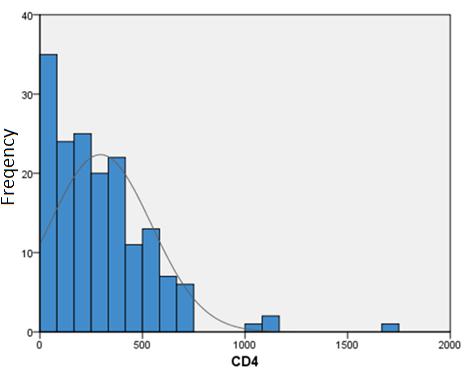 |
Figure 6: Patients' CD4 cells / mm3 histogram |
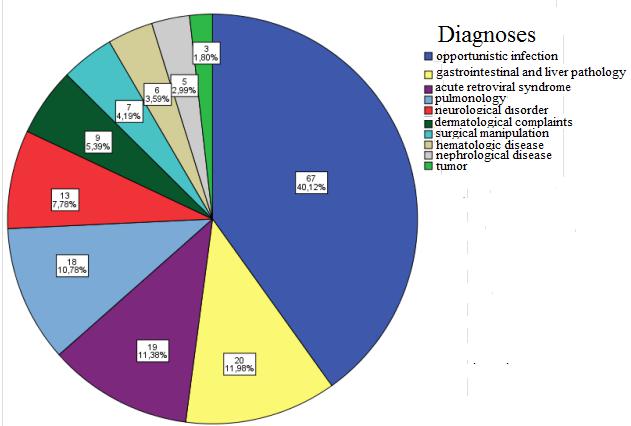 |
Figure 7: Distribution of patients by hospitalization Reasons |
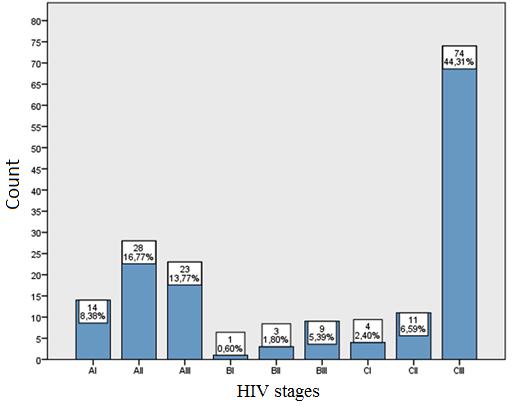 |
Figure 8: Patient distribution by stages of HIV |
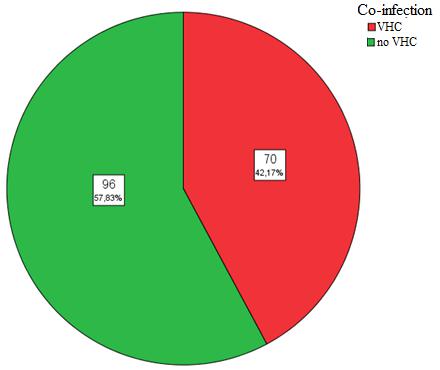 |
Figure 9: Patient distribution by frequent co-infections |
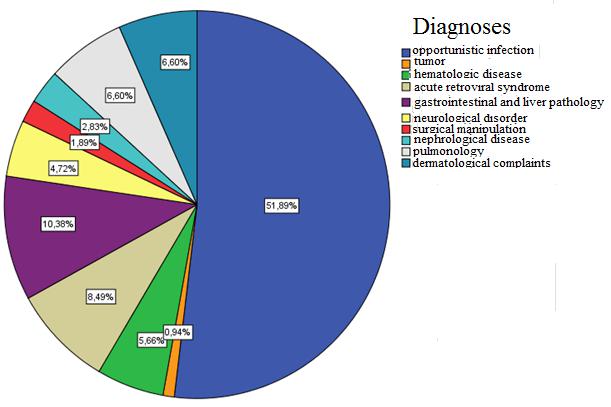 |
Figure 10: Distribution of diagnoses patients with CD4 <350 cell / mm3 |
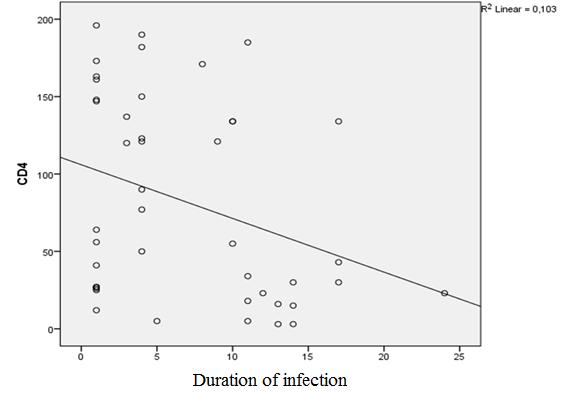 |
Figure 11: CD4 count correlation with patients' infection duration, patients with CD4 <200 cell / mm3and do not get treatment |
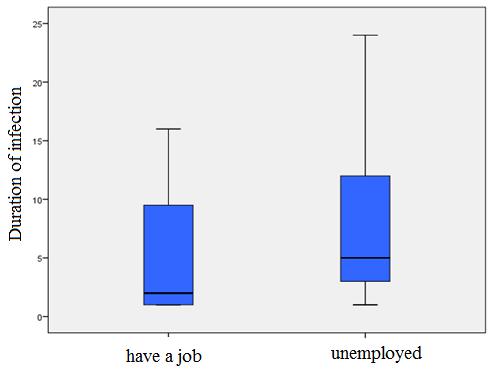 |
Figure 12: Patient distribution according to work and the existence of the average duration of infection |
Diagnose |
(%) |
||
|---|---|---|---|
Pneumocystis Carinii pneumonia |
42 |
||
Wasting syndrome due to HIV |
11 |
||
Candidiasis of esophagus |
15 |
||
Kaposi's sarcoma |
11 |
||
HIV associated dementia |
4 |
||
Disseminated CMV |
4 |
||
Toxoplasmosis of brain |
3 |
||
Disseminated M.avium |
5 |
||
Lymphoma |
4 |
||
Herpes ulcer(s) |
simplex: |
chronic |
1 |
Cryptococcosis of brain |
3 |
||
Cryptosporidiosis |
2 |
||
Tuberculosis |
5 |
||
| Table 1: Data for San Francisco (JID 2002; 186:1019) | |||
CD4 cells |
Infection diseases |
Non-infection diseases |
|---|---|---|
>500/mm3 |
Acute HIV syndrome Candida vaginitis |
Generalized limphadenophaty Polimyositis Aseptic myositis Guillain-Barre syndrome |
200-500/mm3 |
Pneumoccocal and other pneumonies (90) Tuberculosis (90-180) Kaposi's sarcoma (30-150) Herpes zoster (150-170) Candidiasis of esophagus Cryptosporidiosis |
CIN Ca utheri (180) Lymphocitical pneumonitis (100-500) Mononeuritis Anemia Idiophatic trombocitophenic purpur |
<200/mm3 |
P. carinii pneumonia (40-120) Esophageal candidiasis (30-80) Disseminated/chronic herpes simplex (40-110) Toxoplasmosis (20-40) Cryptococcosis (20-60) Disiminēta histoplasmosis(30) Disseminated coccidioidomycosis (40) Chronic cryptosporidiosis (40-130) Progressive multifocal leukoencephalopathy (PML) (40-110) Microsporidiosis (20-100) Miliary/ extrapulmonary tuberculosis (40) CMV disease (10-20) M.avium Complex infection (10-20) |
Weight loss (20-100) B- cell lymphoma (30-60) Cardiomyopathy (25) Peripheral neuropathy (30-100) HIV- associated dementia (20-60) CNS Lymphoma (20) HIV associated nephropathy (20) |
| Table 2: Correlation between HIV infection complications and CD4 cells count | ||
CD4 (cells/mm3) |
Treatment recommendation |
|---|---|
<200 |
Treat irrespective of clinical stage |
200−350 |
Consider treatment and initiate before CD4 count drops below 200 cells/mm |
>350 |
Do not initiate treatment |
| Table 3: Summarizes the immunological criteria for the initiation of 396 ART [23] | |
Female |
% |
Male |
% |
|
|---|---|---|---|---|
IDU |
28 |
16.8% |
34 |
20.4% |
unknown |
42 |
25.1% |
31 |
18.6% |
homosexual way |
0 |
0% |
8 |
4.8% |
heterosexual way |
18 |
10.8% |
6 |
3.6% |
| Table 4: Infection transmission comparison between the sexes | ||||
Have a job |
% |
Unemployed |
% |
|
|---|---|---|---|---|
HCV co-infection |
14 |
25.9% |
56 |
50% |
No HCV co-infection |
40 |
74.1% |
56 |
50% |
| Table 5: HCV infection is the presence of a comparison between patients with or without work | ||||
Have a job |
% |
Unemployed |
% |
|
|---|---|---|---|---|
HCV co-infection |
14 |
25.9% |
56 |
50% |
No HCV co-infection |
40 |
74.1% |
56 |
50% |
| Table 5: HCV infection is the presence of a comparison between patients with or without work | ||||
Have a job |
% |
Unemployed |
% |
|
|---|---|---|---|---|
IDU |
11 |
20% |
51 |
45.5% |
unknown |
29 |
52.7% |
44 |
39.3% |
homosexual way |
5 |
9.1% |
3 |
2.7% |
heterosexual way |
10 |
18.2% |
14 |
12.5% |
Table 6: HIV transmission comparison between patients with or without work |
||||
IDU |
% |
Unknown |
% |
Homose xual way |
% |
Heterosexual way |
% |
|
|---|---|---|---|---|---|---|---|---|
HCV co-infection |
39 |
63.9% |
23 |
31.5% |
1 |
12.5% |
7 |
29.2% |
No HCV co-infection |
22 |
36.1% |
50 |
68.5% |
7 |
87.5% |
17 |
70.8% |
Table 7: HCV infection is the presence of a comparison between the patient HIV transmission modes |
||||||||
Receive therapy |
% |
Common % |
Don’t recive therapy |
% |
common% |
|
|---|---|---|---|---|---|---|
Have a job |
16 |
35.6% |
15.1% |
14 |
23% |
13.2% |
Unemployed |
29 |
64.4% |
27.4% |
47 |
77% |
44.3% |
42.5% |
57.5% |
|||||
Table 8: Therapy existence comparison between patients with CD4 <350 cells / mm3 and employment |
||||||
HCV co-infection |
% |
Common % |
No HCV co-infection |
% |
Common% |
|
|---|---|---|---|---|---|---|
IDU |
15 |
62.5% |
31.9% |
9 |
35.1% |
19.1% |
unknown |
5 |
20.8% |
10.6% |
10 |
43.5% |
21.3% |
homosexual way |
0 |
0% |
0% |
1 |
4.3% |
2.1% |
heterosexual way |
4 |
16.7% |
8.5% |
3 |
13.1% |
6.4% |
51.1% |
48.9% |
|||||
Table 9: Groups of patients with CD4 <350 cell / mm3 and receiving treatment and there is no work-HCV co-infection compared with HIV transmission |
||||||
Have a job |
% |
Common % |
Unemployed |
% |
Common% |
|
|---|---|---|---|---|---|---|
IDU |
2 |
14.3% |
3.6% |
20 |
48.8% |
36.4% |
Unknown |
6 |
42.9% |
10.9% |
15 |
36.5% |
27.3% |
Homosexual way |
2 |
14.3% |
3.6% |
0 |
0% |
0% |
Heterosexual way |
4 |
28.6% |
7.3% |
6 |
14.6% |
10.9% |
Table 10: Transmission and working presence difference in patients with CD4 <350 cell / mm3and opportunistic infections |
||||||
Opportunistic infections |
% |
Not opportunistic infections |
% |
|
|---|---|---|---|---|
HCV co- infection |
26 |
38.8% |
44 |
44.4% |
No HCV co- infection |
42 |
61.2% |
55 |
55.6% |
Table 11: HCV infection is the presence of a comparison between patients with or without opportunistic diseases of HIV |
||||
HCV co- infection |
% |
No HCV co- infection |
% |
|
|---|---|---|---|---|
Opportunistic infections |
26 |
37.1% |
41 |
42.7% |
Tumors |
2 |
2.9% |
1 |
1.0% |
Haematological disorders |
4 |
5.7% |
2 |
2.1% |
Acute Antiretroviral Syndrome |
2 |
2.9% |
17 |
17.7% |
Liver and GIT pathology |
14 |
20.0% |
6 |
6.2% |
Neurological disease |
6 |
8.6% |
7 |
7.3% |
Surgical manipulation |
6 |
8.6% |
1 |
1.0% |
Nephrological disorder |
1 |
1.4% |
4 |
4.2% |
Pulmonology |
6 |
8.6% |
11 |
11.5% |
Dermatologic disease |
3 |
4.3% |
6 |
6.2% |
Table 12: Hospitalization diagnoses of HIV comparison between patients with or without HCV co-infection |
||||
CD4 <200 |
% |
Common % |
CD4 >200 |
% |
Common % |
|
|---|---|---|---|---|---|---|
Opportunistic infections |
40 |
59.7% |
24% |
27 |
42.7% |
16.2% |
Tumors |
0 |
0.0% |
0.0% |
3 |
100% |
1.8% |
Haematological disorders |
3 |
50% |
1.8% |
3 |
50.0% |
1.8% |
acute antiretroviral syndrome |
5 |
26.3% |
3.0% |
14 |
73.7% |
8.4% |
Liver and GIT pathology |
6 |
30.0% |
3.6% |
14 |
70.0% |
8.4% |
Neurological disease |
5 |
38.5% |
3.0% |
8 |
61.5% |
4.8% |
Surgical manipulation |
1 |
14.3% |
0.6% |
6 |
85.7% |
3.6% |
Nephrological disorder |
2 |
40.0% |
1.2% |
3 |
60.0% |
1.8% |
Pulmonology |
4 |
22.2% |
2.4% |
14 |
77.8% |
8.4% |
Dermatologic disease |
4 |
44.4% |
2.4% |
5 |
55.6% |
3.0% |
Table 13: Hospitalization diagnoses of HIV patients in comparison with CD4 cell counts |
||||||






































