Top Links
Journal of Case Reports and Studies
ISSN: 2348-9820
Periodontal Maintenance Therapy and Generalized Aggressive Periodontitis: Brief Review of the Literature and Case Report
Copyright: © 2018 Cobb CM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
This case report presents an unusual variant of generalized aggressive periodontitis that was managed by nonsurgical therapy which included the use of a novel tooth gel. Also, a brief review of the literature is offered as context for this unusual clinical case. The patient, a 40-year old Caucasian female, presented with advanced bone loss involving all teeth, intense inflammation of the attached gingiva and little obvious supra- or subgingival biofilm and dental calculus. Presence of systemic disease, high levels of periodontal microbial pathogens and IL-1α and IL-1β polymorphisms were evaluated and all determined to be negative. Gingival biopsy resulted in a diagnosis of nonspecific inflammation. Treatment consisted of 2- and 3-month intervals for periodontal maintenance appointments and eventually the introduction of an activated edathamil-based tooth gel. Over the subsequent 4-years the patient’s periodontal status stabilized as manifested by no further loss of tooth supporting bone, a reduction in the percentage of periodontal pockets of 4-6 mm depth and a reduction in the percentage of sites exhibiting bleeding upon periodontal probing. The significance of this case report is to emphasize that, even in cases of severe generalized aggressive periodontitis, nonsurgical periodontal therapy can be effective. In addition, it appears that a novel tooth gel containing activated edathamil is effective at reducing gingival inflammation with a concomitant reduction in percent of sites that bleed on periodontal probing.
Keywords: Periodontitis; Aggressive Periodontitis; Edathamil; Inflammation; Mouth Mucosa
Generalized aggressive periodontitis (GAgP) has historically been known by several names: generalized juvenile periodontitis, rapidly progressive periodontitis, and severe periodontitis. The assignment of different names for a disease with an unusual combination of clinical symptoms indicates that GAgP has been viewed, by most clinicians, as a distinctly different disease from chronic periodontitis [1-3].
Clinically, GAgP is characterized by: 1) early age of onset; 2) rapid rate of progression; 3) relatively little clinical inflammation; 4) sparse supra- and subgingival biofilm and dental calculus; and often there is no that has no contributing or significant medical history. Age of onset is the primary characteristic for differentiating between chronic and aggressive periodontitis [2-4]. Familial tendencies have been described and there is a comparatively sparse subgingival biofilm, leading to the impression that the amount of periodontal destruction is not commensurate with the aggregate of dental calculus and biofilm [2,3]. Clinical inflammation may be similar in both chronic periodontitis and GAgP, in which case other factors, such as age of onset and rate of progression, (if it can be determined) assume importance in the differential diagnosis [3]. Two well-known studies are frequently cited as evidence for the differences in rate of disease progression for chronic periodontitis and aggressive periodontitis [5,6]. A 1983 study by Lindhe, et al. determined that an average attachment loss of 0.2 mm/year in untreated disease was typical for the chronic form of periodontitis [5]. In contrast, a 1986 study by Löe, et al. reported a full-mouth average for loss of attachment of 0.46 mm/year in a group of patients that would now be classified as presenting with GAgP [6]. The recent 2017 World Workshop, sponsored jointly by the American Academy of Periodontology and the European Federation of Periodontology, has updated the classification of periodontal and peri-implant diseases and conditions [4]. The new classification is predicated on clinical presentations. The Workshop report stated that all periodontal diseases are infectious in nature and can be classified as either slowly-progressing (i.e., chronic) or rapidly-progressing (i.e., aggressive) [4].
The diversity of the human oral microbiome is remarkable, involving 700+ different species [7,8]. It should not be surprising that periodontal infections are caused by a much more diverse microbiota than previously thought, i.e., something greater than simply a selection of gram-negative anaerobes. Indeed, over 400+ bacterial species have been described as inhabitants of the human periodontal pocket [7]. Aggregatibacter actinomycetemcomitans, a microbe generally found in low numbers in chronic periodontal disease, has often been considered a major etiologic agent in both localized and GAgP [9-12]. However, recently it has been suggested that, although A. actinomycetemcomitans is associated with aggressive disease in younger patients, this appears not the case in older adult subjects [13]. Still other studies have placed less emphasis on A. actinomycetemcomitans and instead suggest that a genetic driven dysbiosis in the subgingival microbial biofilm may be responsible for the rapid and generalized destructive bone loss associated with GAgP [14,15]. In this regard, it is interesting that several authors have noted the similarity of the putative microbial pathogens in GAgP to that of chronic periodontitis [4,16]. The similarity is particularly apparent in the constituent bacteria of the red and orange complexes which are comprised of Gram negative, anaerobic microbial species from the Prevotella, Porphyromonas, Bacteroides, Parvimonas, Fusobacterium, Campylobacter, Tannerella and Treponema taxa, among others [17,18].
Because of familial clustering, patients exhibiting GAgP are frequently considered to have a genetic predisposition to the disease. There is limited evidence supporting the contribution of a few major genes or of multiple small-effects genes; and evidence suggesting gene-gene and gene-environment interaction effects [19]. However, it should be noted that multiple studies have also indicated a genetic predisposition to the development of chronic periodontitis. Thus, it would appear that a genetic risk factor may exist for both diseases but their specific effect on disease expression remains to be determined [14].
It appears that all forms of chronic and aggressive periodontitis are infections in a genetically susceptible host [3]. Risk factors, simply defined, are comprised of environmental and systemic factors that are capable of modifying the clinical course of disease and/or that increase host susceptibility. In their review, Stabholz et al. suggest that the major risk factors for generalized aggressive and chronic forms of periodontitis are similar to the extent there are no remarkable differences between the two diseases [14]. Both forms of periodontitis have a genetic susceptibility component, although the associated gene polymorphisms may be, to some degree, different. Genetic risk factors for both diseases clearly exist, but their effects on disease expression remain to be clarified [14,19]. Armitage and Cullinan have succinctly condensed the conundrum of discerning chronic from GAgP, i.e., “both diseases share many clinical features, but the specific details of the shared features are not necessarily identical in both forms of the disease [4].” The fact remains that chronic and GAgP each presents traits that are sufficiently different such that a discriminating clinician can render an accurate clinical diagnosis. Compared to chronic periodontitis, GAgP is characterized by: 1) early age of onset; 2) rapid rate of progression; 3) comparatively less clinical inflammation; and 4) comparatively less biofilm and dental calculus [5]. Age remains a primary characteristic in differentiating chronic from GAgP [3,4]. Having emphasized potential differences between chronic periodontitis and GAgP, in terms of treatment, it may be that an exact diagnosis is irrelevant to patient care. Both chronic periodontitis and GAgP are likely to be treated in the same manner and respond with similar degrees of success. For example, Deas and Mealey have noted that non-surgical periodontal therapy can be effective in the management of both forms of periodontitis [20].
The following case presentation of a presumptive GAgP is unusual in two respects: 1) the severity of gingival inflammation in the absence of clinically obvious biofilm and dental calculus; and 2) the lack of response to traditional non-surgical therapy until the introduction of a commercially available activated edathamil-based tooth gel.*
The patient, a 40-year old Caucasian female, was referred in February 2009, by a private practice periodontist, to the University of Missouri-Kansas City School of Dentistry, Advanced Education in Periodontics Program. Her chief complaint was “red gums and loose teeth”.
*Livionex Dental Gel, Livionex Inc., Los Gatos, CA 95032
The patient’s medical history is uncomplicated except for a history of seasonal allergic rhinitis for which she takes over-the-counter antihistamines (Cetirizine HCl or pseudoephedrine HCl). Other medications, taken daily, include a multivitamin and supplemental vitamin D2 (400 mg). The patient has never used tobacco or alcohol.
The patient reported allergies to the following drugs: penicillin, ciprofloxacin, cephalexin, levofloxacin and sulfa. Following the initial periodontal examination, the patient was referred to a physician specialist in allergy, rheumatic disease and immunology for confirmation of the reported drug allergies and evaluation for possible systemic disease. The allergy to the penicillin and the quinolone family antibiotics was confirmed. Laboratory tests revealed an elevated level of systemic inflammation as indicated by a hs-CRP (high sensitivity C-reactive protein) level of 2.2 mg but no definitive systemic disease was noted.
The patient had been under continuous care with the local periodontist since 2001 and her past dental history included full-mouth scaling and root planing and osseous surgery, including an osseous graft involving tooth #29. Periodontal maintenance appointments were every 3-4 months during which the patient was very compliant. Oral hygiene was reported as excellent with little to no plaque or calculus. Because of bruxing habit and tooth mobility, she was fitted with a mandibular acrylic mouth guard in 2002. During this time (2001-2009) the gingivae consistently exhibited inflammation characterized by a uniform bright red color, being slightly more intense in the mandible than the maxilla. At the time of referral, the local periodontistated “she has been difficult to maintain and recently, she has a significant increase in bleeding on probing although her oral hygiene is excellent.”
At the February 2009 appointment, the patient stated she had the following teeth extracted at age 16 years in preparation for orthodontic treatment: #17, #21, #28 and #32. According to the patient, the “red gums” started sometime prior to 2000. The following clinical parameters were measured: periodontal probing depth (PD), gingival recession, clinical attachment loss (CAL), bleeding on probing (BOP), and tooth mobility and furcation involvement. The patient’s occlusion and bite guard were also evaluated. A 2008 full-mouth radiographic survey obtained from the local periodontist showed a horizontal pattern of bone loss ranging from approximately 30% to 70%, respectively. Based on the patient’s age of 40 years, apparent rapid progression of disease (versus that normally seen in chronic periodontitis), ≥ 5 mm of clinical attachment loss, and the horizontal pattern of severe bone loss, a diagnosis of generalized aggressive periodontitis was considered. Due to the intense gingival inflammation a differential of benign mucous membrane pemphigoid or allergenic plasma cell stomatitis was also considered as a possible confounding factor.
A comprehensive written and verbal medical history was taken with focus on possible allergens and systemic disease. Multiple swabs from the acrylic mouth guard were cultured on Sabouraud dextrose agar for Candida sp. and found to be negative. An area of mandibular attached gingiva was biopsied. The histologic diagnosis was “non-specific chronic inflammation”. Direct immunofluorescence for fibrinogen, complement C3, IgG, IgA and IgM showed mild reactivity (++). The pattern of reactivity was not diagnostic of any particular disease process. However, the pathologist did note the composition of the inflammatory cell infiltrate was suggestive of a hypersensitivity reaction (Figures 1 and 2).
Subsequent to the biopsy report the patient was referred to an allergist for a comprehensive patch test (including a plastics panel) and a cytoplasmic anti-neutrophil cytoplasmic antibody (C–ANCA) test. The C-ANCA test was to rule out the possibility of a diagnosis of granulomatosis with polyangiitis, a.k.a., Wegener’s granulomatosis. All tests proved negative. Autoimmune disease was deemed unlikely since the oral symptoms were existent for several years and systemic symptoms of an autoimmune disease would have become apparent during this time. Because of its role in calcium and bone metabolism, the patient was referred to her family physician for determination of cholecalciferol (vitamin D3) levels. Laboratory results indicated a serum level of 25 ng/ml which falls within normal limits. Saliva samples were procured and sent to a commercial laboratory† to facilitate DNA probe testing for levels of eleven periodontopathic microbes and detection of levels of IL-1 genetic variations. Interestingly, none of the eleven pathogenic microbes associated with chronic periodontitis (A. actinomycetemcomitans, Campylobacter rectus, Capnocytophaga sp.(gingivalis, ochracea, sputigina), Eikenella corrodents, Eubacterium nodatum, Fusobacterium nucleatum/periodonticum, Parvimonas micra, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, and Treponema denticola) were elevated beyond the “pathogen load threshold”, i.e., the concentration above which patients are considered at increased risk of disease progression. In addition, results were negative for levels of the IL-1α (G4845T) and IL-1β (G4845T) and IL-1β polymorphism (C3954T). These specific polymorphisms are purported to be associated with increased risk of developing periodontitis [21,22].
Initial treatment consisted of the extraction of teeth #1 and #16, an occlusal adjustment and new acrylic bite guard. Definitive scaling and root planing (SRP) of all pockets with PD of ≤ 4 mm following which she was started on low dose doxycycline,‡ 20 mg, twice a day, for 3 months. The patient was then initially placed on a 2-month interval of periodontal maintenance which continued from 2009 through 2012 and then a 3-month interval thereafter. Clinical photographs and radiographs were obtained in 2012 and 2015 (Figures 3 and 4). Comparison of the 2012 and 2015 photographs reveals a marked reduction in gingival inflammation, more so in the maxilla than the mandibular anterior. Interestingly, the only difference in treatment between the years of 2009 and 2014 was the introduction of an activated edathamil-based tooth gel in February 2014. The 2015 radiographic survey showed no improvement in bone levels compared to that of 2012 (Figure 5). However, beginning in 2014, there was a modest and gradual increase in the percentage of PDs that measured ≤ 3 mm with a concomitant decrease in those of 4-6 mm (Figure 6). At the same time interval (2014) there was a notable decrease in the percent of sites that exhibited BOP, i.e., 38% in 2013 to 15% at 2017-18 appointments (Figure 7). Currently, there is no change in PDs or BOP from those recorded in 2015. Consequently, it would appear that the patient’s periodontal status, although far from being ideal, has been relatively stable over the last 4 years.
† MyPerioPath® and MyPerioID PST®, OralDNA® Labs, Eden Prairie, MN 5534.
Destructive periodontal diseases are inflammatory conditions characterized by localized degradation of connective tissue and alveolar bone. Aggressive periodontitis, as opposed to the more common chronic periodontitis, is further characterized by onset at a relatively early age, a rapid rate of progression and a sparse accumulation of supra- and subgingival biofilm and dental calculus. Age of onset and rapid loss of supporting alveolar bone (0.2 mm/year for chronic periodontitis vs. 0.46 mm/year for aggressive periodontitis) are the two major distinguishing features of GAgP [5,6]. Numerous authors have reported successful management of GAgP by nonsurgical treatment and patient compliance to periodontal maintenance therapy at 3-month intervals [23,24]. Although adjunctive systemic antibiotic therapy (Metronidazole or Metronidazole plus Amoxicillin) is reported to provide a clinical benefit in the management of GAgP [25], in this case presentation, the patient’s microbiological evaluation revealed no pathogenic microbes that exceeded moderate to high risk threshold levels. Therefore, in lieu of systemic antibiotics, low dose doxycycline (20 mg) was prescribed to reduce levels of host neutrophil and macrophage derived collagenase production under the assumption that the enzyme may be a contributing factor to the gingival inflammation and aggressive loss of bone. The low dose doxycycline therapy was continued for six months and then discontinued with no apparent change in periodontal status. Interestingly, the decrease in BOP and intensity of gingival inflammation, recorded in 2014 through 2018, appears to be correlated with the use of a tooth gel whose active ingredient is activated edathamil (a.k.a., ethylenediaminetetraacetic acid or EDTA). The concept of using an activated edathamil-based tooth gel is centered on the resulting chelation of calcium.
Calcium has multiple roles in basic cell biology and the inflammatory response. For example, modulation of intercellular Ca2+ concentration is involved in the functional regulation of cells of the immune system [26]. Calcium also participates in regulation of cell differentiation, gene transcription and cell effector functions. Increased intracellular calcium ion levels results from the engagement of immunoreceptors, e.g., T‑ and B‑cell receptors and Fc receptors, as well as chemokine and co‑stimulatory receptors [27].
The involvement of dysregulated lymphocyte Ca2+ signaling in the pathogenesis of autoimmune and inflammatory immune disorders suggests that interference with Ca2+ signaling may be a useful approach to treat autoimmune diseases, such as systemic lupus erythematosus or rheumatoid arthritis, and inflammatory disorders. There is clinically relevant evidence supporting the concept that disruption of calcium dependent cellular reactions can inhibit the inflammatory response. For example, inhibition of the Ca2+‑dependent phosphatase calcineurin combined with adjunctive cyclosporin A and FK506 binding protein is an established therapeutic approach to avert the rejection of allogeneic organ transplants and for the treatment of more severe cases of rheumatoid arthritis, psoriasis or ulcerative colitis [28].
Calcium ions also play a role in the binding and adhesion of the bacteria in developing biofilms and between the pioneer bacterial colonizers and the host interface [29]. In the case of biofilm binding to teeth, the host interface is comprised of a salivary glycoprotein surface precipitate, generally known as the acquired pellicle. Previous studies have reported that formulations containing activated edathamil achieve disruption of plaque without adversely affecting the enamel surface microstructure [30]. In addition, clinical trials have shown that activated edathamil tooth gel significantly reduces both dental biofilm (plaque) and gingival inflammation [31,32]. Traditional clinical practice has advocated extraction of teeth with a questionable prognosis due to severe bone loss, thereby avoiding further loss of local bone volume and eventual involvement of neighboring teeth. This practice remains pervasive in spite of the fact that at least one study has shown that bone regeneration is possible with periodontal therapy with no negative effects on immediately adjacent teeth [33]. Indeed, Graetz, et al. reported results of a 15-year study in which patients with GAgP, 88.2% of teeth initially diagnosed as having a questionable prognosis and 59.5% of teeth with a hopeless prognosis, survived 15-years during regular periodontal maintenance care [33]. Thus, it would seem that either non-surgical or surgical treatment have the potential to preserve the natural dentition of patients diagnosed with GAgP with the caveat that patients are compliant with long-term periodontal maintenance therapy. Furthermore, as shown in the current case report, it would appears that use of activated edathamil tooth gel offers a significant clinical benefit in terms of reductions in gingival inflammation, bleeding on probing and accumulation of tooth associated microbial biofilms.
The diagnostic differentiation between generalized chronic vs. generalized aggressive periodontitis can be surprisingly difficult. The aggressive disease, as the name would imply, is characterized by rapid loss of tooth supporting bone and the relatively early age of onset. This case report presents an unusual clinical presentation of severe GAgP that was successfully managed by nonsurgical therapy with short-interval periodontal maintenance therapy and the adjunctive use of a novel tooth gel containing a calcium chelator consisting of activated edathamil.
 |
| Figure 1: Hematoxylin and eosin stained gingival biopsy specimen showing an intact stratified squamous epithelium with parakeratosis. Note the elongated epithelial rete pegs between which there is a dense infiltrate of inflammatory cells (arrows). Original magnification of x100 |
 |
| Figure 2: High magnification view of the inflammatory cell infiltrate noted in Figure 1. Predominant cell types are plasma cells and lymphocytes (arrows). There is notable exocytosis of lymphocytes into the epithelium. Very few neutrophils were observed, thus the histologic diagnosis of chronic nonspecific inflammation. Original magnification of x400 |
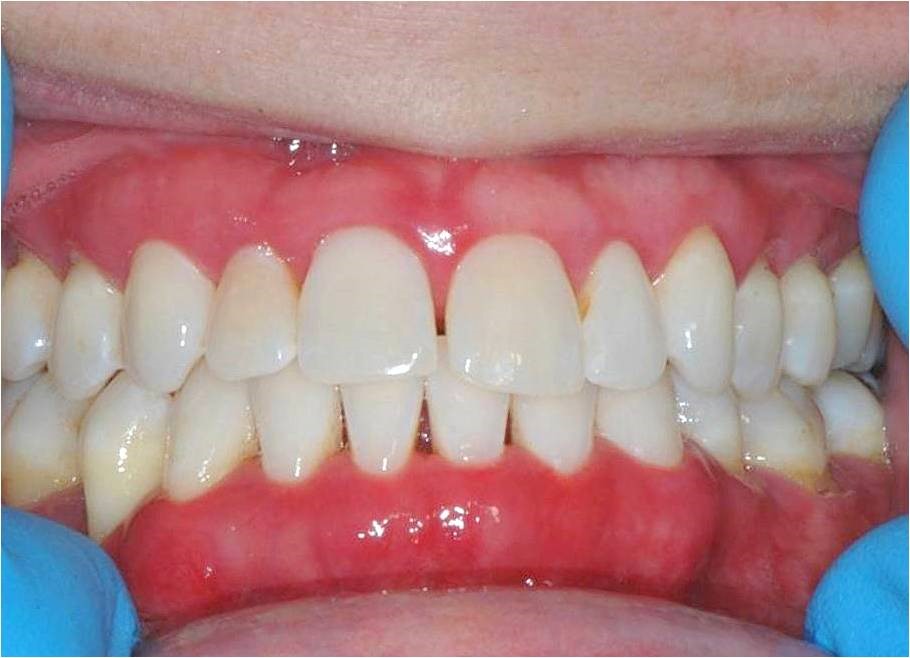 |
| Figure 3: Anterior view taken in 2012 showing intense gingival inflammation and lack of visualdental calculus and biofilm accumulation |
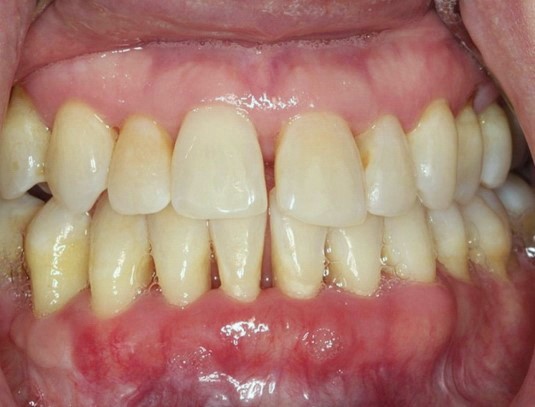 |
|
Figure 4:Anterior view taken in 2015 showing decrease in visual inflammation of the gingiva. Note that the maxillary gingiva is near normal in color while there is residual but mild inflammation of the mandibular anterior keratinized gingiva |
 |
|
Figure 5:Full-mouth radiographic survey taken in 2012 showing severe loss of supporting bone involving all teeth. The predominant pattern of bone loss is horizontal with isolated areas of developing vertical or angular loss. Such a bone loss pattern, at a relatively young age, is typical for a diagnosis of generalized aggressive periodontitis |
| Figure 6: Percent change in probing depth over time (n = 156 sites). Vertical axis is a percent scale showing the percent change in periodontal probing depth. The horizontal axis shows the percent of probing depths ≤ 3 mm and 4-6 mm by year (2009-2018) |
| Figure 7: Percent change in BOP over time represented by the highest score for each year of periodontal maintenance appointments. Vertical axis is a percent scale and horizontal axis is the year. Patient started using the activated edathamil-based tooth gel in February 2014 |






































