Top Links
Journal of Case Reports and Studies
ISSN: 2348-9820
Macrocystis of the Lung in a Preterm Neonate: Case Report
Copyright: © 2017 De Bernardo G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Background: Thoracic ultrasonography has been used to evaluate pulmonary parenchyma and the macrocystis of the lung in a preterm with congenital cystic adenomatoid malformation type I. The images gained have been important for diagnosis and therapeutic strategies in our case.
Case summary: An infant was prematurely born with congenital cystic adenomatoid malformation type I. The chest X-ray and computerized tomography showed a great opaque area in the entire right lung, we considered performing a pneumonectomy. This opaque area hampered the expansion of left lung. After draining the macrocystis by ultrasonography-guided puncture, it was possible considering and performing the lobectomy due to the reduction of the volume of macrocystis. During follow up at 4 months, the infant did not show complications and the chest X-ray revealed the reduction of volume of the dense area in parenchyma of right lung.
Conclusion: In a congenital cystic adenomatoid malformation, health care providers should try to expand the health lung before of considers a surgical operation. The evaluation of the macrocystis with computerized tomography and ultrasonography allowed choosing the therapeutic strategy. If multiple lobes are affected, pneumonectomy should be performed while if lesion is confined in one lobe, a lobectomy should be executed.
Keywords: NICU; Pneumonectomy; Lobectomy; Lung; Case report
List of Abbreviations: CCAM: Congenital Cystic Adenomatoid Malformation; GA: Gestational Age; CT: Computerized Tomography; US: Ultrasonography; FiO2: Fraction of Inspired Oxygen; MAP: Mean Airway Pressure; ΔP: Delta Pressure; Hz: Hertz; PIP: Peak Inspiratory Pressure; PEEP: Positive End Expiratory Pressure; RR: Respiratory Rate
Congenital cystic adenomatoid malformation (CCAM) is characterized by cystic masses in the lung and an abnormal proliferation of the terminal respiratory bronchioles. It was described by Stocker, et al. in 1977 and occurs rarely with an incidence of 1:25,000 to 1:35,000 pregnancies [1]. There are 3 types of CCAM:
• CCAM type I characterized by one or more frequently several cystis greater than 20mm
• CCAM type II characterized by multiple cystis smaller than 10mm
• CCAM type III characterized by microcystis or solid lesion, non-cystic.
The most common treatments are: intra-uterine surgery, sclerotherapy, segmentectomy, lobectomy and pneumonectomy [2-6]. We are reporting a case CCAM type I. During prenatal age a thoraco-abdominal shunting was inserted in to the utero to reduce the volume of the macrocystis. Due to premature birth, the thoraco-abdominal shunting was removed, the macrocystis didn’t decrease, and surgery couldn’t be avoided. Computerized tomography (CT) and chest X-ray showed that all lobes were compromised by macrocystis so it was considered pneumonectomy. During ultrasonography evaluation, macrocystis were revealed in sub pleural and drained. The right lung parenchyma expanded and the volume of macrocystis reduced then lobectomy was considered. At 4 months follow up there were not complications reported.
During the second trimester, a prenatal ultrasonography (US) revealed numerous cystis filled with liquid in the upper half of right lung of a female infant Caucasian (Figure 1A). The largest macrocyst was of 14x11mm (Figure 1B). It was suspected a CCAM. For this reason, the baby was monitored on a regular basis by prenatal US. During the third trimester, prenatal US showed hydrothorax, hydropericardium, abdominal ascites, a hypoplastic left lung and the largest macrocystis grew up to 24x33mm (Figure 1C). A thoraco-amniotic drainage was placed to drainage and reduce the volume of cyst and the prenatal US in the 7th month proved that the largest macrocyst decreased to 25x22mm (Figure 1D). Unexpectedly, the labour began and the baby was born at 29+1/7 weeks of gestational age (GA) by Caesarean section in emergency in III level Hospital. She was intubated and ventilated with mask–bag with FiO2=0.21. Apgar score was 4I, 7V and 9X. Her body weight was 1400gr, length 40.7cm and head circumference was 26cm. Due to respiratory distress the infant was subjected to a mechanical ventilation by high frequency oscillations, this procedure is recommended for hypoplastic lungs, with MAP=14cmH20, ΔP=18cmH20, Hz=10 and FiO2=0.40. At 2 days of life the thoraco-amniotic drainage inserted in uterus during pregnancy was removed. At 6 days of life, the baby showed a right tension pneumothorax therefore a chest drain was inserted. At 7 days, she was transferred to an incubator with synchronized intermitted mandatory ventilation with FiO2=0.25, PIP=20cmH2O, PEEP=5cmH2O, RR=38b/min at Santobono-Pausilipon, III level Hospital and reference center for neonatal surgical diseases. In neonatal intensive care unit, mechanical ventilation was continued with the same modality. The clinical condition of the infant remained stable enough allowing the assessment and developments of macrocystis without performing an emergency surgery. A chest X-ray was preformed showing the right lung completely opaque (Figure 2A). CT detected a voluminous mass heterogeneously hypodense in the right lung that hampered the expansion of left lung (Figure 2B, C and D). Probably, the respiratory distress was caused both from the prematurity and the left lung depressed by mass. It was considered a pneumonectomy of the right lung because cystis were present in all lobes. In order to avoid pneumonectomy it was performed a pulmonary US to evaluate the size of macrocystis and their location. It revealed that there were two subpleural macrocystis in the middle lobe and the largest was 26x19mm (Figure 3A), also lines B were not visible (Figure 3B). At 9 days, after birth the two largest macrocystis were drained by US-guided puncture. At 13 days, it was repeated US-guided puncture, the macrocystis decreased, and the lines B appeared (Figure 3C). At 14 days, the infant underwent to surgical operation to remove only the middle right lobe containing the malformation. The lobectomy was performed with a good outcome. Histological examination confirmed CCAM type I, according to the Stoker’s classification [1]. After surgical operation the chest X-ray detected a reduction of opaque area in the right lung and the expansion of the left lung (Figure 4A). At 4 months after birth the chest X-ray showed attenuation and reduction of volume and dense area in the parenchyma of the right lung also the smallest cystis regressed spontaneously (Figure 4B). There were not complications until discharge at the 5 months.
Prenatal US is important to evaluate the size of cystis because it can have a predictive value for prognosis. The microcystis that appears as solid echogenic mass tends to regress spontaneously from 26 to 28 weeks of gestation. However, cystis greater than 5mm do not regress spontaneously as fluid accumulates within the cysts [7]. The increase in volume of the cystis can cause the compression of the surrounding parenchyma and the displacement of the mediastinum. If cystis do not decrease spontaneously, an intra-uterine surgery must be considered. In these cases, a thoraco-amniotic shunting can reduce the volume of cystis to avoid pulmonary hypoplasia [7]. The prenatal US of our patient showed numerous cystis in the right lung. The biggest macrocyst was 14x11mm and grew up to 24x33mm. A thoraco-amniotic shunting was performed but as the cysts were decreasing the infant was prematurely born. Prenatal management, it is also practiced sclerotherapy, embolization of collateral vessels, radiofrequency ablation and maternal steroid therapy. Sclerotherapy and embolization occlude feeding vessel of cystis and reduce its volume until recovery while radiofrequency ablation was used to ablate a large cyst but it resulted in fetal death due to haemorrhage [5]. The maternal steroid therapy instead is performed at 30 weeks of GA to mature the lungs due to risk of premature delivery caused by the invasive procedure or to reduce the volume of cystis [4]. The use of artificial ventilation can determinate the grow of cystis but if the intubation of the healthy main bronchus is done by conventional ventilation or high frequency ventilation, this procedure can cause the expansion of healthy lung [8,9]. We did not perform the selective intubation of the healthy main bronchus because the patient was preterm. She was subjected to synchronized intermittent mandatory ventilation with hope to expand the right lung parenchyma but without success. The postnatal management includes surgery operation in symptomatic patients by segmentectomy, lobectomy or pneumonectomy. If possible, the lobectomy should always be preferred to pneumonectomy. The pneumonectomy is inevitable if multiple lobes are affected and when the lesion is not confined. However if the lesion is confined in one lobe, a lobectomy it should be performed. Otherwise, if the lesion comprises multiple lobes it should be taken into account segmentectomy combined with lobectomy [10]. In our case report, initially we have considered a pneumonectomy of right lung because the cystis were present in all the lobes and made opaque the right lung to CT and to the chest X-ray. In order to avoid a pneumonectomy that seemed inevitable, the two largest macrocystis were localized and drained by US guided puncture in neonatal intensive care unit. We performed this procedure because the macrocystis were subpleural and did not decrease further. Initially lines B were absent due to macrocystis fluid filled but after drainage we observed their presence, which revealed the expansion of parenchyma lung so much that we considered performing a lobectomy [11]. In a case report of CCAM type I, the macrocystis were drained to reduce them. The US guided puncture of macrocystis was attempt but failed due to the fact that the macrocyst was filled with air and liquid. Then the transthoracic drain was inserted by CT images of chest so the macrocystis were reduced. A possible complication of this procedure is bronchopleural fistula and major leak of air [12]. In another case report in which radiofrequency ablation was used to ablate a large congenital cystic malformation resulted in fetal death, presumed to be caused by a haemorrhage into the necrotic mass [13]. This case report would suggest cautious use of any procedure, either ante-or postnatal, which results in infarction of the mass [5]. Health care providers should try to expand the heath lung before of consider a surgical operation. The evaluation of the macrocystis with computerized tomography and ultrasonography allow choosing the best therapeutic strategy. The strength of our work is that we evaluated macrocystis by US directly in NICU and performed a lobectomy of the middle lobe with a good outcome. The limitation is that US guided puncture of macrocystis is possible only if these are sub pleural. At 4 months, the infant did not show complications, as a matter of fact there was a reduction of volume of the dense area in parenchyma of right lung.
In a congenital cystic adenomatoid malformation, health care providers should try to expand the health lung before of considers a surgical operation. The evaluation of the macrocystis with computerized tomography and ultrasonography allowed choosing the therapeutic strategy. If multiple lobes are affected, pneumonectomy should be performed while if lesion is confined in one lobe, a lobectomy should be executed.
We thank family for giving us written informed consent and Patrizia Aversa for writing assistance.
“Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.”
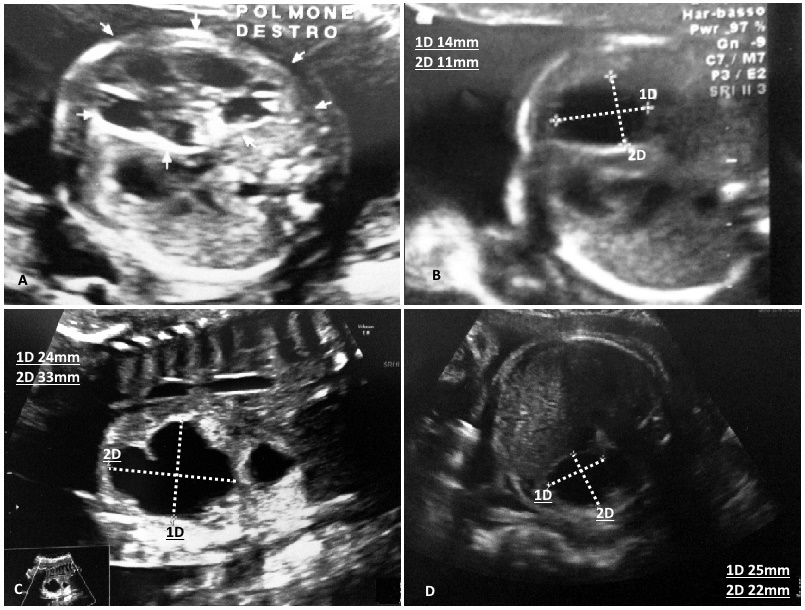 |
| Figure 1: (A) During second trimester, US showed numerous cystis; (B) The largest macrocyst was 14x11mm; (C) At third trimester, macrocyst grew up to 24x33mm; (D) After operation macrocyst decreased to 25x22mm |
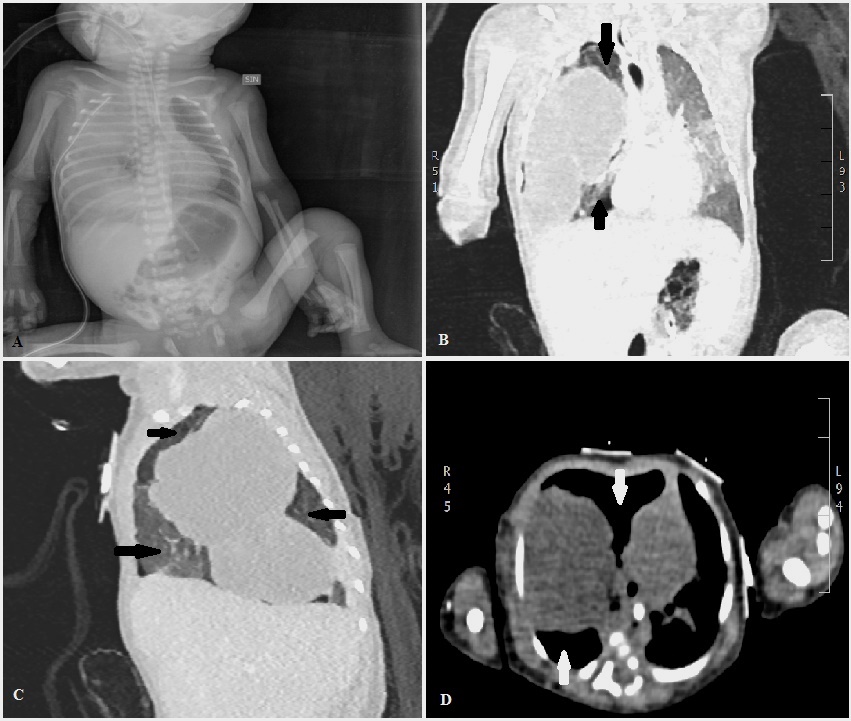 |
| Figure 2: (A) At 7 days, chest X-ray revealed a right lung completely opaque; (B) CT detected a voluminous mass in the right lung that hampered the expansion of left lung; (C and D) The section sagittal and axial showed that all lobes were compromised but there were areas without cystis (one arrow) |
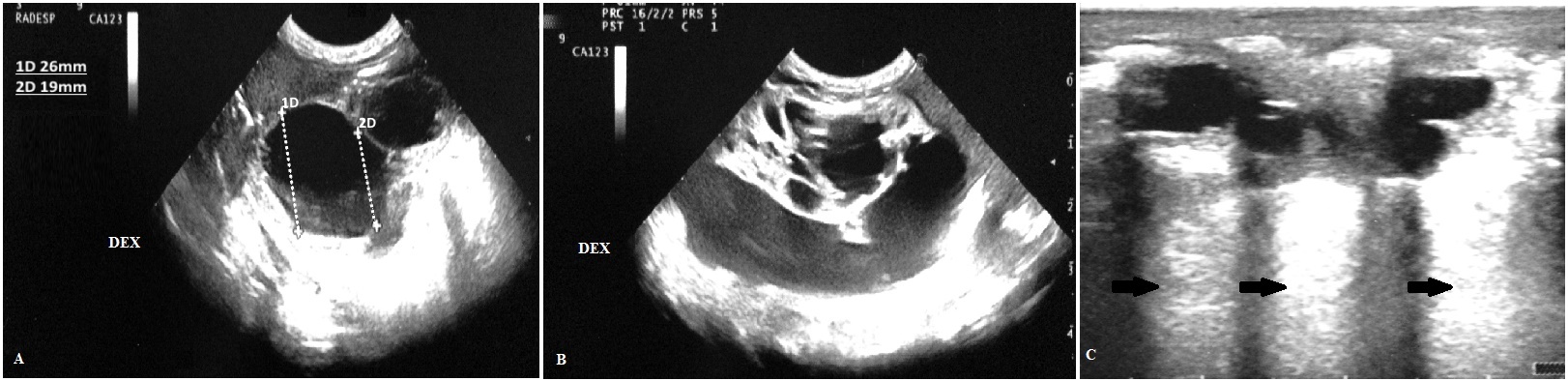 |
| Figure 3: (A) At 8 days, the pulmonary US revealed that macrocyst was 26x19mm; (B) The macrocystis did not allow seeing the lines B; (C) At 9 days, after the second US guided puncture the macrocystis decreased and allowed seeing the lines B (one arrow). There was a great extension of lines B, especially these air artifacts were compact and clear up from mamillary line in direction apical-caudal until the anterior axillary line in the medial-lateral direction |
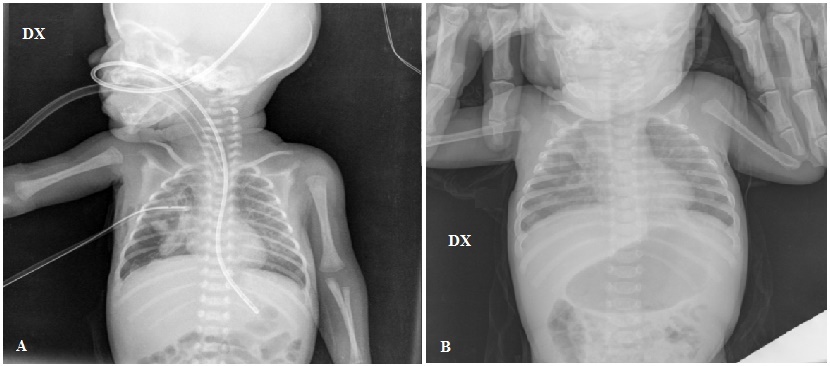 |
| Figure 4: (A) Chest X-ray after surgical operation showed a reduction of opaque area in the right lung and the expansion of the left lung; (B) After 4 months chest X-ray revealed a further attenuation and reduction of opaque area in the right lung, also the smallest cystis regressed spontaneously |






































