Top Links
Journal of Antibiotics Research
ISSN: 2574-5980
in-vitro Antibacterial / Antifungal Screening of 2-Chloroquinoline Scaffold Derivatives
Copyright: © 2015 Kumar S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
A series of differentiated 2-chloroquinoline derivatives (3-26) having various spacer groups between 2-chloroquinoline and aryl or heteroaryl ring were synthesized by chemical reactions involving nucleophilic addition, nucleophilic substitution, esterification and cyclization. All the synthesized compounds were analyzed by one or more technique such as FTIR, 1H-NMR, 13C-NMR and mass spectrometry for their structural confirmation. The derivatives were screened in-vitro for their ability to inhibit the growth of various strains of fungi and bacteria at concentration ranging from 6.25, 12.5, 25, 50, 100, 200 to 400 μg/ml. The results of in-vitro screening unveiled that among all the compounds tested, compounds 21 showed potent antibacterial activity and its MIC was found to be in the range of 12.5 μg/ml. In addition molecular docking studies were also performed on PDB ID (3G75) to predict the mode of action of the potent compound 21.
Keywords: Antibacterial; Antifungal; 2-chloroquinoline; Docking; Oxadiazoles
The emergence of multi-drug resistance strains of bacteria and fungi such as Methicilin resistant Staphylococcus aureus (MRSA), Vancomycin resistant enterococcus (VRE) and Fluconazole resistant Candida species have made treatment of infectious diseases difficult and over the time have become a serious medical problem [1-5]. The mechanism of resistant is continuously evolving in pathogenic bacteria to currently used antimicrobials [6-8]. The search for new antimicrobial agents have been an important and challenging task for medicinal chemists [9-11] and discovery of novel and potent antimicrobial agents is still a best way to combat this situation [12-13].
Development new antimicrobial drug involving chemical modification of existing drugs or class of drug has come up with astound results in the field of drug discovery [14]. Some newly approved drugs or investigational drug which utilizes this strategy has allowed finding of more active and safe compounds with wide spectrum activity. Figure 1 presents some of potential investigational molecules which are in either phase II or Phase III of clinical trial derived from existing NCE's [15]. Searching for structure with propitious bioactivity we focused our attention on quinoline and its congeners which had revealed as diverse and potent antibacterial, antifungal, antimalarial, anticancer drugs antimalarial drugs and are under continuous evaluation for the development of potent bioactive molecules [16].
Some of the recent chemical modifications quinoline include Bedaquiline (R207910) which has shown extraordinary activity against both drug susceptible and drug-resistant strains of M. tuberculosis, exhibiting MIC values of 30-120 ng/ml, [17]. Laquinimod is an experimental immunomodulator drug and it is currently under investigation for oral treatment of multiple sclerosis (MS) [18]. GSK 299423 is an investigational compound which has shown potent activity against antibiotic-resistant strains of bacteria such as Staphylococus aureus, including methicillin resistance S. aureus (MRSA) and against gram-negative bacteria like E. coli, Pseudomonas, Klebsiella and Acinetobacter [19] (Figure 2). Fascinated by multifarious bioactivity of quinoline various researchers and scientists are still engaged in developing potent molecule based on quinoline such as Saeed et al. [20] have reported the synthesis of conformationally constrained Analogs of N-Substituted Piperazinylquinolones tested for antimicrobial activity. Likewise various 4-pyrazolyl-N-(hetero)arylquinoline were prepared by Nilesh et al. [21] and observed that some of the compounds were more or equipotent against most of the employed strains than commercially available drugs. Impelled by these observations and in continuation of our research for bioactive molecules based on 2-chloroquinoline system [22-24], we address here synthesis and in-vitro antimicrobial activity of some newer differentiated 2-chloroquinoline derivatives.
Melting points were determined by the open capillary method with electrical melting point apparatus and are uncorrected. IR spectra were recorded as KBr (pellet) on Bio Rad FT-IR spectrophotometer and 1H and 13C-NMR spectra were recorded on Bruker DPX 300 MHz spectrophotometer using DMSO-d6 or CDCl3 as a NMR solvent. Mass spectra (MS-ESI) were recorded on a JEOL-AccuTOF JMS-T100LS mass spectrometer and elemental analysis on Vario-EL III CHNOS- Elemantar analyzer. Thin Layer Chromatography (TLC) was performed to monitor progress of the reaction and purity of the compounds, spot being located under iodine vapors or UV-light.
The starting material 2-chloro-3-formyl-quinoline 1 and 2-chloro-3-formyl-6-methylquinoline 2 were prepared according to the literature method [25].
To a solution of 2-chloro-3-formyl-quinoline (0.96 g, 0.005 mol) 1 or 2-chloro-3-formyl-6-methylquinoline 2 (1.03 g, 0.005 mol) in 20 ml of absolute ethanol, equimolar amount of isonicotinic acid or benzoic acid hydrazide (0.68 g, 0.005 mol) was added and the mixture refluxed for 2-4 h. On cooling solid was obtained which was filtered, washed with hot methanol, dried and recrystallized from ethanol and DMF mixture to give final compounds.
N'-[(2-Chloroquinolin-3-yl)methylidene]benzohydrazide 3: Yield: 82 %; m.p.: 220-223 ºC; Anal. Calcd for C17H12ClN3O; C 65.92; H 3.90; N 13.57 %. Found; 65.71, H 3.93, N 13.64 %; IR (KBr) cm-1: 3260 (N-H), 1650 (C=O), 1625 (C=N), 1579 (C=C), 755 (C–Cl).1H-NMR (300 MHz, DMSO-d6); δ 7.54-7.63 (m, 3H, Ar-H), 7.68-7.73 (t, 1H, H-6, J = 7.39 Hz), 7.85-7.90 (t, 1H, H-7, J = 7.44 Hz), 7.95-7.99 (m, 3H, Ar-H), 8.22-8.25 (d, 1H, H-8, J = 7.08 Hz), 8.82 (s, 1H, H-4), 8.94 (s, 1H, CH=N), 12.22 (s, 1H, CONH). 13C-NMR (75 MHz, DMSO-d6); δ 126.1, 126.8, 127.6, 127.7, 128.5, 128.9, .131.9, 133.0, 135.6, 142.7, 147.1, 148.4, 157.1, 169.2 (C=O).
N'-[(2-Chloroquinolin-3-yl)methylidene]pyridine-4-carbohydrazide 4: Yield: 87 %; m.p.: >280 ºC; Anal. Calcd for C16H11ClN4O: C 61.84; H 3.57; N 18.03 %. Found C 61.97, H 3.56, N 18.06 %; IR (KBr) cm-1: 3365 (N-H), 1697 (C=O), 1629 (C=N), 1595 (C=C), 750 (C-Cl).1H-NMR (300 MHz, DMSO-d6) ; δ 7.66-7.71 (t, 1H, H-6, J = 7.42 Hz), 7.86-7.89 (m, 3H, Ar-H), 7.96-7.99 (d, 1H, H-5, J = 8.33 Hz), 8.19-8.22 (d, 1H, H-8, J = 8.08 Hz), 8.80-8.84 (m, 3H, Ar-H), 8.91 (s, 1H, CH=N), 11.49 (s, 1H, CONH).
N'-[(2-Chloro-6-methylquinolin-3-yl)methylidene]benzohydrazide 5: Yield: 81 %; m.p.: 190-192 ºC; Anal. Calcd for C18H14ClN3O: C 66.77; H 4.36; N 12.98 %. Found : C 66.51; H 4.38; N 12.94 %; IR (KBr) cm-1: 3261 (N-H), 1655 (C=O), 1627 (C=N), 1589 (C=C), 759 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): δ 2.51 (s, 3H, CH3), 7.57-7.64 (m, 3H, Ar-H), 7.70-7.73 (d, 1H, H-7, J = 8.49 Hz), 7.85-7.88 (d, 1H, H-8, J = 8.52 Hz), 7.97-8.01 (m, 3H, Ar-H), 8.82 (s, 1H, H-4), 8.93 (s, 1H, CH=N), 12.26 (s, 1H, CONH). MS (ESI) m/z: 310.09 [M+], 312.09 [M+2].
N'-[(2-Chloro-6-methylquinolin-3-yl)methylidene]pyridine-4-carbohydrazide 6: Yield: 86 %; m.p.: >280 ºC; Anal. Calcd for C17H13ClN4O; C 62.87; H 4.03; N 17.25 %. Found C 62.69; H 4.01; N 17.28 %; IR (KBr) cm-1: 3278 (N-H), 1673 (C=O), 1629 (C=N), 1588 (C=C), 756 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 2.51 (s, 3H, CH3), 7.67-7.70 (d, 1H, H-7, J = 8.28 Hz), 7.83-7.87 (m, 3H, Ar-H), 7.99 (s, 1H, H-5), 8.77-8.80 (m, 3H, Ar-H), 9.02 (s, 1H, CH=N), 11.23 (s, 1H, CONH).
A mixture hydrazones (3-6) (0.001 mol), chloramines-T (1.14 g, 0.005 mol) and 10 ml of abs. ethanol taken in a round bottom flask and refluxed for 6-8 hr. The progress of the reaction was monitored on TLC. After word the reaction mixture was poured in water and extracted with ether. The combined extract was washed with water and dried over anhydrous sodium sulphate and concentrated under reduced pressure [26].
2-Chloro-3-[5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl]quinoline 7: Yield: 67 %; m.p.: >300 ºC; Anal. Calcd for C16H9ClN4O: C 62.25; H 2.94; N 18.15 %. Found; C 62.49: H 2.92; N 18.21 %; IR (KBr) cm-1: 1629 (C=N), 1595 (C=C), 750 (C-Cl).1H-NMR (300 MHz, DMSO-d6) ; δ 7.62 (t, 1H, H-6, J = 7.2 Hz), 7.69-7.81 (m, 2H, H-5 and H-7), 7.93-8.04 (m, 3H, Ar-H), 8.09 (s, 1H, H-4), 8.80-8.87 (m, 3H, Ar-H).13C-NMR (75 MHz, DMSO-d6); δ120.7, 121.0,126.6, 127.0, 129.9, 130.5, 131.2, 137.2, 141.7, 144.7, 148.0, 148.7, 149.0, 164.5, 165.7. ESI-MS: m/z 309.12 [M+], 311.12 [M+2].
2-Chloro-6-methyl-3-[5-(pyridin-4-yl)-1,3,4-oxadiazol-2-yl]quinoline 8: Yield: 55 %; m.p.: > 300 ºC; Anal. Calcd for C17H11ClN4O: C 63.26: H 3.44; N 17.36 %. Found: C 63.03; H 3.46; N 17.42 %; IR (KBr) cm-1: 1629 (C=N), 1588 (C=C), 756 (C-Cl). 1H-NMR (300 MHz, DMSO-d6): δ 2.51 (s, 3H, CH3), 7.68 (d, 1H, H-7, J = 8.2 Hz), 7.89-8.02 (m, 3H, Ar-H), 8.05 (s, 1H, H-5), 8.77-8.84 (m, 3H, H-4 & 2 x pyridine).13C-NMR (75 MHz, DMSO-d6); δ 20.8, 121.4, 122.0, 126.9, 128.2, 130.9, 131.3, 133.1, 135.9, 142.9, 144.2, 148.3, 148.6, 148.9, 164.7, 165.4. MS (ESI) m/z: 322.14 [M+], 324.14 [M+2].
2-(2-Chloroquinolin-3-yl)-5-phenyl-1,3,4-oxadiazole 9: Yield: 64 %; m.p.: 265-267 ºC; Anal. Calcd for C18H12ClN3O: C 66.35; H 3.28; N 13.65 %. Found: C 66.54, H 3.30, N 13.70 %; IR (KBr) cm-1: 1637 (C=N), 1585 (C=C), 749 (C–Cl). 1H-NMR (300 MHz, DMSO-d6); δ 7.18 (d, 1H, Ar-H, J = 7.1 Hz), 7.69-7.73 (m, 2H, Ar-H), 7.81-7.84 (m, 2H, Ar-H), 7.98 (d, 1H, H-5, J = 8.3 Hz), 8.22-8.26 (m, 2H, Ar-H), 8.79 (s, 1H, H-4).13C-NMR (75 MHz, DMSO-d6); δ 21.43 (CH3), 125.88, 126.36, 127.93, 128.08, 128.74, 129.13, 131.53, 133.26, 134.10, 135.93, 143.41, 146.84, 148.15, 159.09, 165.2, 165.9.
2-(2-Chloro-6-methylquinolin-3-yl)-5-phenyl-1,3,4-oxadiazole 10: Yield: 60 %; m.p.: 250-252 ºC; Anal. Calcd for C18H12ClN3O; C 67.19, H 3.76, N 13.06. Found : C 67.43, H 3.78, N 13.11 %; IR (KBr) cm-1: 1622 (C=N), 1597 (C=C), 751 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): δ 7.20 (d, 1H, Ar-H, J =7.8 Hz), 7.65 (d, 1H, H-7, J = 7.4 Hz), 7.81-7.85 (m, 2H, Ar-H), 8.01 (s, 1H, H-5), 8.22 (d, 1H, Ar-H, J =7.3 Hz), 8.79 (s, 1H, H-4). ESI-MS: m/z 321.17, 323.17.
To a solution of compound 1 or 2 (0.01 mol) in absolute methanol, solid sodium borohydride (0.45 g, 0.012 mol) was added portion wise over a period of 30 min. with constant stirring at room temperature. After that solvent was evaporated under reduced pressure and the residue was triturated with water and the crystalline product was filtered, washed with water and dried. The product was recrystallized from methanol.
2-Chloro-3-(hydroxymethyl)-quinoline 11: Yield: 86 %; m.p.: 160-162 ºC; Anal.Calcd for C10H8ClNO: C, 62.03, H 4.16, N 7.23. Found: C 62.20, H 4.14, N 7.27 %; IR (KBr) cm-1: 3340 (O–H), 1614 (C=C), 1592 (C=N), 765 (C–Cl). 1H-NMR (300 MHz, DMSO-d6): δ 4.77 (s, 2H, CH2), 5.45 (s, 1H, OH, D2O-exchangeble), 7.53-7.58 (t, 1H, H-6, J = 7.0 Hz), 7.67-7.72 (t, 1H, H-7, J = 6.9 Hz), 7.83-7.86 (d, 1H, H-5, J = 7.5 Hz), 7.97-8.00 (d,1H, H-8, J = 8.0 Hz), 8.36 (s, 1H, H-4). 13C-NMR (DMSO-d6, 75 MHz): δ 59.9 (CH2), 126.4, 127.0, 127.4, 127.7, 129.9, 133.8, 135.7, 146.0, 148.3. MS m/z: 194. (M). 196 (M+2).
2-Chloro-3-(hydroxymethyl)-6-methylquinoline 12: Yield: 82 %; m.p.: 172-174 ºC; Anal. Calcd for C11H10ClNO: C 63.62, H 4.85, N 6.75. Found: C 63.78, H 4.86, N 6.79 %; IR (KBr) cm-1: 3340 (OH), 1595 (C=N), 1615 (C=C), 751 (C–Cl). 1H-NMR (300 MHz, CDCl3) δ: 2.52 (s, 3H, CH3), 4.66 (s, 2H, CH2), 5.45 (bs, 1H, OH, D2O-exchangeble), 7.54- 7.57 (m, 2H, Ar-H), 7.92 (d, 1H, H-8, J = 7.74 Hz), 8.06 (s, 1H, H-4). 13C-NMR (CDCl3, 75 MHz) δ : 18.94 (CH3), 57. 8 (CH2), 126.1, 126.9, 127.8, 130.5, 131.9, 135.4, 142.0, 148.0. MS m/z: 208 (M+), 210 (M+2).
To a solution of 11 or 12 (0.005 mol) in pyridine (10.0 mL) was slowly added benzoyl chloride (0.7 g, 0.005 mol) or p-tuloloyl chloride (0.77 g, 0.005 mol) at room temperature. After stirring for 10 min, the mixture was allowed to warm at room temperature and maintained for 2 h. The mixture was then diluted with cold water (50 mL), the solid product obtained was washed repeatedly to remove pyridine. The dried product was than recrystallized from ethanol.
(2-chloroquinolin-3-yl) methyl benzoate 13: Yield: 88 %; m.p.: 135-137 ºC; Anal. Calcd for C17H12ClNO2 : C, 68.58; H, 4.06; N, 4.70 % Found : C, 68.58; H, 4.06; N, 4.70 %; IR (KBr) cm-1: 1728 (C=O), 1620 (C=C), 1595 (C=N), 1120 (C-O), 754 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 5.06 (s, 2H, CH2), 7.34-7.42 (m, 3H, Ar-H), 7.48-7.55 (m, 3H, Ar-H), 7.69-7.77 (m, 2H, Ar-H), 8.01 (d, 1H, H-8, J = 7.8 Hz), 8.14 (s, 1H, H-4).13C-NMR (CDCl3, 75 MHz): δ 64.6 (CH2O-), 125.9, 127.2, 127.6, 128.7, 129.0, 129.5, 130.1, 131.4, 132.8, 136.3, 146.0, 152.2, 169.2 (C=O). MS (ESI) m/z: 297.12 [M+] 299.12 [M+2].
(2-chloroquinolin-3-yl) methyl 4-methylbenzoate 14: Yield: 88 %; m.p.: 170-171 ºC; Anal. Calcd for C18H14ClNO2: C, 69.35; H, 4.53; N, 4.49 %. Found; C, 69.16; H, 4.55; N, 4.52 %. IR (KBr) cm-1: 1724 (C=O), 1613 (C=C), 1590 (C=N), 1118 (C-O), 758 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 2.26 (s, 3H, CH3), 5.02 (s, 2H, CH2), 7.22 (d, 2H, H-3'& 5', J = 7.0 Hz), 7.51-7.58 (m, 3H, Ar-H ), 7.71-7.79 (m, 2H, Ar-H), 8.03 (d, 1H, H-8, J = 7.4 Hz ), 8.10 (s, 1H, H-4).13C-NMR (75 MHz, CDCl3) δ: 21.4 (CH3), 64.4 (CH2O), 124.8, 126.4, 127.1, 127.0, 128.6, 129.7, 130.2, 130.8, 131.5, 135.8, 137.1, 143.9, 150.4, 169.4.
(2-chloro-6-methylquinolin-3-yl) methyl benzoate 15: Yield: 88 %; m.p.: 166-167 ºC; Anal. Calcd for C18H14ClN2O: C, 69.35; H, 4.53; N, 4.49. Found:C, 69.59; H, 4.55; N, 4.53 %; IR (KBr) cm-1: 1726 (C=O), 1627 (C=C), 1597 (C=N), 1123 (C-O), 754 (C–Cl). 1H-NMR (300 MHz, CDCl3) δ: 2.52 (s, 3H, CH3), 5.01 (s, 2H, CH2), 7.39-7.45 (m, 3H, Ar-H), 7.55-7.67 (m, 4H, Ar-H,), 7.98 (d, 1H, H-8, J = 7.6 Hz), 8.09 (s, 1H, H-4). 13C-NMR (75 MHz, CDCl3) δ: 18.9 (CH3), 64.7 (CH2O), 125.2, 126.5, 127.2, 127.8, 128.4, 129.5, 130.1, 130.6, 132.1, 136.8, 138.0, 143.9, 151.4, 169.7. MS (ESI) m/z; 311.08 [M+], 313.08 [M+2].
(2-chloro-6-methylquinolin-3-yl) methyl 4-methylbenzoate 16: Yield: 88 %; m.p.: 189-191 ºC; Anal. Calcd for C19H16ClN2O : C, 70.05; H, 4.95; N, 4.30 Found C, 70.23; H, 4.97; N, 4.25 %. IR (KBr) cm-1 : 1730 (C=O), 1613 (C=C), 1590 (C=N), 1117 (C-O), 758 (C–Cl).1H-NMR (300 MHz, CDCl3) δ: 2.27 (s, 3H, Ph-CH3), 2.51 (s, 3H, CH3), 5.03 (s, 2H, CH2), 7.25 (d, 2H, H-3' & 5', J = 7.2 Hz), 7.52-7.61 (m, 4H, Ar-H,), 7.97 (d, 1H, H-8, J = 8.0 Hz), 8.09 (s, 1H, H-4).13C-NMR (75 MHz, CDCl3) δ: 18.7 (CH3), 21.4 (CH3), 64.9 (CH2O), 112.9, 118.0, 126.3, 127.3, 127.7, 129.3, 130.4, 132.2, 135.9, 137.0, 145.3, 147.1,148.8.
To a solution of compound 11 or 12 (0.01 mol) in dry benzene, SOCl2 (1.55 g, 0.013 mol) was added and the mixture refluxed for 4 hr. Solvent was evaporated under reduced pressure and the residue was dissolved in ether, washed with 10% NaHCO3 and twice with water. Dried over Na2SO4 and concentrated in vacuvo to give a residue which was crystallized from methanol.
3-(chloromethyl)-2-chloroquinoline 17: Yield: 83 %; m.p.: 116 ºC; Yield 80%; mp. 116 °C; Anal.Calcd for C10H7Cl2N: C, 56.63, H, 3.33; N, 6.60. Found: C, 56.46; H, 3.31; N, 6.63 %; IR (KBr) cm-1: 1620 (C=C), 1590 (C=N), 754 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 4.82 (s, 2H, CH2), 7.54-7.59 (t, 1H, H-6, J = 7.2 Hz), 7.71-7.76 (t, 1H, H-7, J = 7.5 Hz), 7.81-7.83 (d, 1H, H-5, J = 7.9 Hz), 8.00-8.03 (d,1H, H-8, J = 8.3 Hz), 8.26 (s, 1H, H-4). 13C-NMR (CDCl3, 75 MHz): δ 43.03 (CH2), 126.91, 127.38, 127.45, 128.12, 128.89, 130.87, 138.60, 147.12, 149.51. MS: m/z 212 (M+), 214 (M+2).
2-Chloro-3-(chloromethyl)-6-methylquinoline 18: Yield: 88 %; m.p.: 140 ºC; Yield 85 %, m.p. 140 °C; Anal. Calcd for C11H9Cl2N : C, 58.43; H, 4.01; N, 6.19. Found: C, 58.30; H, 4.03; N, 6.22 %; IR (KBr) cm-1 : 1620 (C=C), 1595 (C=N), 759 (C–Cl), 1H-NMR (300MHz, CDCl3) δ: 2.53 (s, 3H, CH3), 4.87 (s, 2H, CH2), 7.55-7.57 (m, 2H, Ar-H), 7.92 (d, 1H, H-8, J = 7.5 Hz), 8.17 (s, 1H, H-4). 13C-NMR (CDCl3, 75 MHz) δ: 21.48 (CH3), 43.17 (CH2), 125.91, 127.12, 127.93, 129.06, 135.76, 141.13, 147.16, 149.31. MS: 226 (M+), 228 (M+2).
To a mixture of compound 3 (0.003 mol) and sulphanilamide/ p-aminophenol (0.003 mol) in 20 mL of absolute ethanol, 1 mL of triethylamine (TEA) was added and refluxed for 12-15 h. After completion of the reaction, content of the flask reduced to half and left overnight. The crystalline mass obtained was filtered off, washed with water, dried and recrystallized from ethanol to give 19-22.
4-{[(2-Chloroquinolin-3-yl)methyl]amino}benzenesulfonamide 19: Yield: 71 %; m.p.: 182-184 ºC; Anal.Calcd for C16H14ClN3O2S, C,55.25; H, 4.06; N, 12.08. Found: C, 55.41; H, 4.07; N, 12.13 %; IR (KBr) cm-1: 3298 (N–H), 1619 (C=C), 1599 (C=N), 1340 (S=O), 1029 (C–N), 736 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 4.37 (bs, 1H, NH, D2O-exchangeble), 4.60 (s, 2H, CH2), 6.65 (d, 2H, H-2' & 6', J = 8.0 Hz), 6.82 (bs, 2H, SO2NH2), 7.15 (d, 2H, H-3' & 5', J = 7.8 Hz), 7.55 (t, 1H, H-6, J = 7.4 Hz), 7.67-7.76 (m, 2H, Ar-H), 8.03 (d, 1H, H-8, J = 8.2 Hz), 8.10 (s, 1H, H-4). 13C-NMR (CDCl3, 75 MHz): δ 43.1 (CH2), 112.4, 112.6, 125.9, 126.7, 127.2, 127.9, 128.2, 129.3, 129.8, 131.3, 132.0, 146.0, 151.5, 152.8. MS (ESI) m/z: 347.11 [M+], 349.11 [M+2].
4-{[(2-Chloro-6-methylquinolin-3-yl)methyl]amino}benzenesulfonamide 20: Yield: 66 %; m.p.: 201-203 ºC; Anal. Calcd for C17H16ClN3O2S: C, 56.43; H, 4.46; N, 11.61. Found:C, 56.26; H, 4.48; N, 11.68 %; IR (KBr) cm-1 : 3290 (N–H), 1619 (C=C), 1589 (C=N), 1337 (S=O), 1028 (C–N), 742 (C–Cl). 1H-NMR (300 MHz, CDCl3) δ : 2.52 (s, 3H, CH3), 4.30 (s, 1H, NH, D2O-exchangeble), 4.62 (s, 2H, CH2), 6.70 (d, 2H, H-2'& 6', J = 7.8 Hz), 6.84(bs, 2H, SO2NH2 7.17 (d, 2H, H-3' & 5', J = 7.3 Hz), 7.57-7.66 (m, 2H, H-5 & H-7), 7.95 (d, 1H, H-8, J = 7.8 Hz), 8.10 (s, 1H, H-4). 361, 363
4-((2-Chloroquinolin-3-yl)methyl)amino)phenol 21: Yield: 62 %; m.p.: 221-223 ºC; Anal. Calcd for C16H13ClN2O: C, 67.49; H, 4.60; N, 9.84.Found: C, 67.66; H, 4.62; N, 9.89 %; IR (KBr) cm-1: 3387 (N–H), 3452 (O-H) 1633 (C=C), 1593 (C=N), 1040 (C–N), 752 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 4.35 (s, 1H, NH, D2O-exchangeble), 4.59 (s, 2H, CH2), 6.62 (d, 2H, H-2' & 6', J = 7.6 Hz), 6.89 (H-3' & 5', J = 7.8 Hz), 7.53-7.57 (t, 1H, H-6, J = 7.4 Hz), 7.71-7.79 (m, 2H, H-5 and H-7), 8.07 (d, 1H, H-8, J = 8.0 Hz), 8.11 (s, 1H, H-4), 11.52 (s, 1H, OH).13C-NMR (CDCl3, 75 MHz): δ 44.4 (CH2), 115.8, 116.2, 116.8, 126.6, 126.9, 127.35, 127.8, 129.5, 130.8, 135.7, 142.5, 146.2, 147.2, 152.0. MS (ESI) m/z: 284.14 [M+], 286.14 [M+2].
4-((2-Chloro-6-methylquinolin-3-yl)methylamino)phenol 22: Yield: 68 %; m.p.: 254 ºC; Anal. Calcd for C17H15ClN2O: C, 68.34; H, 5.06; N, 9.38. Found:C, 68.56; H, 5.07; N, 9.44 %. IR (KBr) cm-1: 3408 (N–H), 3459 (O-H), 1632 (C=C), 1598 (C=N), 1040 (C–N), 752 (C–Cl). 1H-NMR (300 MHz, CDCl3) δ : 2.52 (s, 3H, CH3), 4.38 (s, 1H, NH, D2O-exchangeble), 4.62 (s, 2H, CH2), 6.66 (d, 2H, H-2' & 6', J = 7.4 Hz), 6.91 (d, 2H, H-3' & 5'J = 7.6), 7.99 (d, 1H, H-8, J = 7.5 Hz), 8.10 (s, 1H, H-4) 11.62 (s, 1H, OH).
Sodium hydroxide (0.132 g, 0.0033 mol) was slowly added over 5 min to a stirred solution of 2-mercaptobenzimidazole (0.21 g, 0.0014 mol) or 6-nitro-2-mercaptobenzimidazole (0.28 g, 0.0014 mol) in ethanol (20 mL). Compound 17 or 18 (0.0016 mol) was slowly added to this solution at 0 ºC and stirred for 12-14 hrs at room temperature. The completion of the reaction was monitored on TLC and after that solvent was removed under reduced pressure, the residue was poured into 10% NaHCO3 solution and extracted with ethyl acetate. The organic layer was dried over Na2SO4, and concentrated. The residue was crystallized from methanol [27].
3-[(1H-benzimidazol-2-ylsulfanyl)methyl]-2-chloroquinoline 23: Yield: 73 %; m.p.: 211-213 ºC; Anal. Calcd for C17H12ClN3S; C, 62.67; H, 3.71; N, 12.90. Found:C, 62.78; H, 3.73; N, 12.97 %. IR (KBr) cm-1: 1625 (C=C), 1599 (C=N), 1091 (C-S-C), 757 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 4.69 (s, 2H, CH2), 7.27-7.30 (m, 2H, Ar-H), 7.52-7.60 (m, 3H, Ar-H), 7.69-7.76 (m, 2H, H-7 and 5), 8.03 (d,1H, H-8, J = 8.3 Hz), 8.13 (s, 1H, H-4).13C-NMR (CDCl3, 75 MHz): δ 38.0 (CH2), 116.1, 116.8, 124.8, 125.0, 126.6, 127.2, 127.7, 128.1, 129.3, 131.2, 137.9, 138.3, 145.6, 148.4, 151.0. MS (ESI) m/z: 325.10 [M+], 327.10 [M+2].
2-Chloro-3-{[(6-nitro-1H-benzimidazol-2-yl)sulfanyl]methyl}quinoline 24: Yield: 60 %; m.p.: 238-240 ºC; Anal. Calcd for C17H11ClN4O2S ; C, 55.06; H, 2.99; N, 15.11. Found:C, 55.27; H, 2.97; N, 15.19 %; IR (KBr) cm-1: 1630 (C=C), 1590 (C=N), 1088 (C-S-C), 751 (C–Cl). 1H-NMR (300MHz, CDCl3) δ: 2.51 (s, 3H, CH3), 4.70 (s, 2H, CH2S), 7.25-7.28 (m, 2H, Ar-H), 7.58-7.67 (m, 4H, Ar-H), 7.98 (d, 1H, H-8, J = 7.8 Hz), 8.10 (s, 1H, H-4).13C-NMR (CDCl3, 75 MHz): δ 38.3 (CH2), 113.2, 115.7, 118.5, 125.8, 126.2, 126.7, 127.5, 129.3, 131.1, 136.7, 139.0, 144.7, 145.3, 150.0, 152.3.
3-[(1H-benzimidazol-2-ylsulfanyl)methyl]-2-chloro-6-methylquinoline 25: Yield: 64 %; m.p.: 196-198 ºC; Anal. Calcd for C18H14ClN3S; C, 63.62; H, 4.15; N, 12.36. Found: C, 63.51; H, 4.17; N, 12.43 %; IR (KBr) cm-1: 1631 (C=C), 1594 (C=N), 1095 (C-S-C), 752 (C–Cl). 1H-NMR (300 MHz, CDCl3): δ 4.72 (s, 2H, CH2), 7.21-7.23 (d, 1H, Ar-H, J = 7.3 Hz), 7.57-7.62 (m, 3H, Ar-H), 7.67-7.75 (m, 2H, H-7 and 5), 8.05 (d,1H, H-8, J = 8.0 Hz), 8.10 (s, 1H, H-4).
2-Chloro-6-methyl-3-{[(6-nitro-1H-benzimidazol-2-yl)sulfanyl]methyl}quinoline 26: Yield: 59 %; m.p.: 209-211 ºC; Anal. Calcd for C18H13ClN4O2S ; C, 56.18; H, 3.40; N, 14.56. Found:C, 56.42; H, 3.41; N, 14.61 %; IR (KBr) cm-1: 1620 (C=C), 1589 (C=N), 1093 (C-S-C), 750 (C–Cl). 1H-NMR (300MHz, CDCl3) δ: 2.52 (s, 3H, CH3), 4.70 (s, 2H, CH2S), 7.19-7.21 (d, 1H, Ar-H, J = 7.5 Hz), 7.57-7.69 (m, 4H, Ar-H), 8.01 (d, 1H, H-8, J = 7.2 Hz), 8.08 (s, 1H, H-4).
The newly synthesized compounds (3-26) were tested against a panel of bacterial strains such as Escherichia coli (NCTC, 10418), Staphylococcus aureus (NCTC, 65710), Pseudomonas aeruginosa (NCTC, 10662) and fungal strains viz. Aspergillus niger (MTCC, 281), Aspergillus flavus (MTCC, 277), Monascus purpureus (MTCC, 369), Penicillium citrinum (NCIM, 768) by cup-plate method. Potato dextrose agar (PDA) and nutrient agar were used as culture medium for antifungal and antibacterial activity respectively. Normal saline with tween 80 (0.01%) was used to make suspension of fungal and bacterial spore for lawning. Fifty milliliters of PDA medium was poured into each petri dish (15 cm diameter). Five ml of the spore suspension was spread over the solid agar medium and plates were dried in incubator at 37° for 1 hr. Using an agar punch, wells were made on these seeded agar plates and solutions of test compounds in DMSO at conc. range of 6.25, 12.5, 25.0, 50, 100 and 200 μg/ml were added into each well, labeled previously. A control was also prepared using solvent DMSO. The Petri plate were prepared in duplicate and incubated at 30 °C for 72 hr for fungi and 37 °C for 24 hr for bacteria. Antifungal activity was determined by measuring zone of inhibition and the minimum inhibitory concentration (MIC) was noted by seeing the lowest concentration of the test drug at which there was no visible growth. Activity of each compound (3-26) was compared with standard Fluconazole and Ciprofloxacin and results have been summarized as MIC (average zone of inhibition of two reading in millimeter) in Table 1.
The crystal structure of bacterial DNA gyrase (PDB code: 3G75, Resolution-2.30 Å)was retrieved from Protein Data Bank (PDB) and was utilized for molecular docking studies .Protein was prepared with the Protein Preparation Wizard in Maestro using options: bond orders were assigned, hydrogen atoms were added, formal charges were treated and water molecules were deleted. Hydrogen bonding network was then optimized using the exhaustive sampling option and the protein was minimized to an RMSD limit from the starting structure of 0.3 Å using the Impref module of Impact with the OPLS_2005 force field. Prepared protein structure was used to generate Glide scoring grids for the subsequent docking calculations. Docking grids was generated with the default settings in Glide using the co-crystalized ligand (B48)to define the centre of the grid box (20×20×20 Å). Default parameters were used and no constraints were included during grid generation. The three dimensional coordinates of the most potent compound 21 was generated using Maestro module of Schrodinger. Ligands were prepared using LigPrep 2.6 with Epik 2.4 to expand protonation and tautomeric states at 7.0 ± 2.0 pH units and energy was minimized using the OPLS 2005 force field. The docking calculations were performed by Glide XP docking.
The physiochemical properties important for ADME (Absorption, Distribution, Metabolism and Excretion) considerations were predicted using QikProp 3.6 (Schrodinger) that calculates properties like molecular weight, molecular volume, no. of H-bond donors, no. of H-bond acceptors, polar surface area, Q Plog Po/w (Predicted octanol/water partition coefficient) and violations related to Lipinski's "Rule of 5" and Jorgensen's "Rule of 3" to filter out compounds with clear-cut undesirable properties. The prerequisite was to neutralize the compounds before being used by QikProp. The neutralization step was carried out using Lig prep after which all the hits from both the approaches were processed for calculation of ADME properties.
The various 2-chloroquinolines were synthesized as per the scheme outlined in Figure 3. Different routes were adopted for the synthesis of target compounds starting from the common intermediates 1 and 2. The 1,3,4-oxadiazole derivatives (7-10) were synthesized by cyclisation of hydrazones (3-6) of isonicotinic acid hydrazide (INH) or benzoic acid hydrazide with intermediate 1 and 2 using chloramine-T as catalyst in refluxing ethanol. The 2-chloro-3-formylquinoline and 2-chloro-3-formyl-6- methylquinoline (1, 2) were further reduced to alcohol (11, 12) using solid NaBH4 in methanol and subsequent reaction of 11 or 12 with benzoyl or p-methyl benzoyl chloride in pyridine affords various benzoate derivatives (13-16). The chlorination of compounds (11, 12) with SOCl2 in dry benzene afforded intermediates 3-(chloromethyl)-2-chloroquinoline (17, 18) and their successive nucleophilic substitution reaction with sulphanilamide or p-aminophenol in absolute ethanol in the presence of organic base triethylamine (TEA) gave 2-chloroquinolinyl amines (19-22). While various 1H-benzimidazol-2-ylsulfanyl)methyl (23-26) derivatives were prepared by reacting intermediate (17, 18) with 2-mercaptobenzimidazole or 2-mercapto-6-nitrobenzimidazole in ethanol in presence of base NaOH.
The structure of diverse 2-chloroquinoline derivatives was elucidated by combined use of IR, 1H and 13C-NMR and mass spectral data. The presence of the 1,3,4-oxadiazole unit in compounds (7-10) was supported by the appearance of two quaternary signals of (C-2, C-5) at δ value 164.5 and 166.7 ppm in 13C-NMR spectrum of compound 7. This was further supported by mass spectrum of compound 7 (m/z 309.12). The synthesis of compounds (13-16) was achieved by reacting quinoline carbinol derivatives (11, 12) with benzoyl chloride in pyridine. The formation of benzoate derivatives were established by locating characteristics peak of -CH2OCO- which was observed in the range at δ value 5.01-5.06 ppm integrating for two protons in 1H-NMR. In 13C-NMR this particular function was observed at δ value 64.6 ppm for compound 13. In IR spectra the characteristics C=O and C-O band for compounds (13-16) were observed at 1724 -1730 and 1117-1123 cm-1 respectively. The synthesis of secondary amines (19-22) of sulphanilamide/ p-aminophenol was identified by locating -CH2NH- function in spectral data. The 1H-NMR signal due methylene of -CH2NH- was observed at δ value 4.59-4.62 ppm, while the NH proton was resonated at 4.30-4.38 ppm as singlet or broad singlet. The synthesis was further confirmed by mass spectrometry in which molecular ion peak was registered at m/z 347.11 (M+) and M+2 peak at 349.11 for compound 20. The synthesis of compounds (23-26) was established by identifying the characteristics -CH2S- peak in NMR. In 1H-NMR spectra of compounds (23-26) the signal due methylene proton of -CH2S- group was resonated at δ value 4.69-4.72 integrating for two protons. While in 13C-NMR, the methylene carbon was identified at δ 38.0 for compound 23. All these observations confirm successful synthesis of compounds.
The diversified 2-chloroquinoline derivatives were tested for their antibacterial activity against gram positive and gram negative bacterial strains viz. Escherichia coli NCTC 10418, Staphylococcus aureus NCTC 65710, Pseudomonas aeruginosa NCTC 10662 and antifungal activity against three fungal strains viz. C albican, A. flavus, A. niger using cup-plate method at conc. range of 6.25, 12.5, 25, 50, 100, 200 and 400 μg/ml [28,29].
Results of antibacterial screening are presented in Table 1 as MIC the conc. at which no visible growth was observed (zone of inhibition in mm). The quinolinyl hydrazones (3-6) and there corresponding oxadiazoles (7-10) exhibited variable effect on the growth of bacterial strains. The hydrazones and oxadiazoles of INH, compounds (3, 5, 7 and 8) exhibited MIC of 12.5 to 50 μg/ml against test strains and among these compound 7 and 8 showed MIC of 12.5 μg/ml against the E. coli. While hydrazones and oxadiazoles of benzoic acid hydrazide (4, 6, 9 and 10) showed MIC in the range of 50 to 200 μg/ml. The difference in the MIC within these analogues (3-10) may be attributed to presence of INH residue which itself is a potent antimycobacterial agent. The intermediate compound 11 and 12 showed moderate antibacterial (MIC 50 to 100 μg/ml) activity and their corresponding ester (13-16) turns from moderately active to weakly active (MIC 100 to 200 μg/ml). The chloromethyl intermediate (17 and 18) were also showed weak activity which was observed at (MIC 200 μg/ml) against the test bacterial strains. While their corresponding secondary amines of sulphanilamide (19, 20) and p-aminophenol (21, 22) was comparatively more active in inhibiting the growth of the bacteria (MIC 12.5 to 25 μg/ml). Among the 2-mercaptobenzimidazole derivatives (23-26), the nitro derivatives were more active against the all the bacterial strains and there MIC was observed in the range of 25-50 μg/ml.
The antifungal activity quinolinyl hydrazones (3-6) and there corresponding oxadiazoles (7-10) derivatives was found to be weak as their MIC were observed in the range of 100 to 200 μg/ml against test strains. The compound 11 and 12 also exhibited weak antifungal activity while there ester analogue (13-16) were slightly more active than the parent compound and there MIC were observed in between 50 to 100 μg/ml. The antifungal activity of chloromethyl derivatives of 2-chloroquinoline (17 and 18) was found in the range of 50 to 100 μg/ml. The quinolinyl amine derivatives of sulphanilamide and p-amniophenol (19-22) showed antifungal activity in the range of 25-100 μg/ml. The benzimidazole derivatives (23-26) showed moderate antifungal activity against the test strain C. albicans, A. niger and A. flavus was and there MIC observed at 25 to 50 μg/ml.
To understand the mechanism of action underlying activity of most active compound 21, we proceeded to examine the interaction of compound 21 with bacterial DNA gyrase (PDB code: 3G75) [30]. All docking runs were carried out as per Glide XP Docking protocol in Schrodinger 9.4 [31,32]. The XP Glide score obtained for compound 21 was found to be -7.62. Figure 4 and Figure 5 shows the binding mode of compound 21 interacting with DNA gyrase and revealed that amino acids ASP57, GLU58, ASH81,ILE51, ILE175, VAL79, ILE102, ILE86 and PRO87 located in the binding pocket played vital roles in the interaction of compound 21 with the enzyme. The hydroxyl substituent at the distal phenyl ring and NH group acts as H-bond donar and formed H-bond network with the amino acid residue ASP57 and GLU58 at 1.57 and 2.01 Å respectively.One nitrogen atom of quinoline nucleus providedadditional H-bond with ASH81 at 1.32 Å. The hydrophobic interactions with ILE51, ILE175, VAL79, ILE102, ILE86 and PRO87 further stabilized the compound in the active site of bacterial DNA gyrase.
The ADME (Absorption, Distribution, Metabolism and Excretion) properties are crucial determinants for the successful development of new drugs. Unfavorable ADME properties can lead to rejection of a drug in the later stages of drug process [33]. All the compounds synthesized, were further processed for ADME, Lipinski's "Rule of 5" and Jorgensen's "Rule of 3" using QikProp tool of Schrodinger which is built using experimental details of 710 compounds including 500 drugs and heterocyclic compounds. The QikProp properties obtained for all the compounds are listed in Table 2 [34]. QikProp calculates properties like molecular weight, molecular volume, no. of H-bond donors, no. of H-bond acceptors, polar surface area, QPlogPo/w (Predicted octanol/water partition coefficient) and violations related to Lipinski's "Rule of 5" [35] and Jorgensen's "Rule of 3" [36] to filter out compounds with clear-cut undesirable properties. Compounds that satisfy Lipinski's "Rule of 5" are considered drug-like and compounds with fewer (and preferably no) violations of Jorgensen's "rule of 3", are more likely to be orally available. All the compounds showed excellent ADME properties and passed Lipinski's "Rule of 5" having no violations. In addition, all compounds except 6, 16, 23-26 also passed Jorgensen's "rule of 3", which showed that they have potential of 100 % orally bioavailable. The excellent ADME property of these hits makes them promising candidates for future development as antimicrobial agents.
Impelled by the diverse potential of quinoline derivatives, a series of diversified 2-chloroquinoline derivatives was synthesized and evaluated for antibacterial and antifungal activity. Among the screened derivatives, compound 21 (4-((2-Chloroquinolin-3-yl)methylamino)phenol) was found to be the most potent antibacterial ligand having the MIC of 12.5 μg/ml against E. coli and S. aureus. Against P. auroginosa the MIC was found to be 25 μg/ml. Molecular docking studies were also performed to further investigated the interaction of ligand 21 with active sites of bacterial DNA gyrase (PDB ID 3G75). The XP glide docking simulation studies exhibited that compound 21 forms three hydrogen bonds with the residue ASP 57, GLU58, ASH 81. The in-vitro studies coupled with computational studies suggest that compound 21 is promising candidate for further exploitation as lead molecules against bacterial infection.
The authors are to Jamia Hamdard for providing necessary facility. Thanks are due to IIT Delhi and CDRI, Lucknow, for recording spectral data.
 |
| Figure 1: Chemical tailoring or chemical remodeling of existing antibacterial drugs classes, showing development of Gatifloxacin or Moxifloxacin from Nalidixic acid and investigational molecules PA-824 and OPC-67683 from Metronidazole etc |
 |
| Figure 2: Chemical structures of some investigational quinoline containing antimicrobial molecules |
 |
| Figure 3: Route of synthesis of various 2-chloroquinoline derivatives compounds (3-26).Reagent and conditions: (a) INH or benzoic acid hydrazide, abs. EtOH, reflux (b) chlormine-T, ethanol/reflux (c) NaBH4 /MeOH, stirring (d) benzoyl chloride/p-methyl benzoyl chloride, pyridine (e) SOCl2, benzene reflux (f) sulphanilamide/p-aminophenol, TEA, ethanol reflux (g) 2-mercaptobenzimidazole/NaOH, ethanol, reflux |
 |
| Figure 4: 2-Dimentional (2D) diagram showing hydrogen bonding interaction of compound 21 with active sites of enzyme DNA gyrase (PDB ID 3G75) |
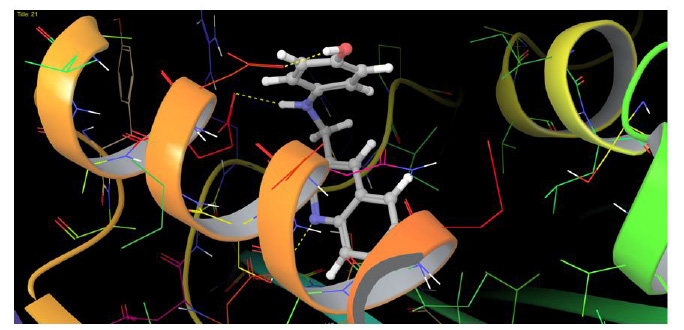 |
| Figure 5: 3-Dimentional (3D) diagram showing hydrogen bonding interaction of compound 21 with active sites of enzyme DNA gyrase (PDB ID 3G75) |
| Compd. No | R | R1/R2/R3 | MIC (zone of inhibition in mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Antibacterial activity | Antifungal activity | |||||||
| E. coli | S. aureus | P. aurogiosa | C. albican | A.flavus | A. niger | |||
| 3 | H | 4-Pyridinyl | 25 (6.5) | 50 (7.5) | 50 (6.5) | 200 (6.0) | 100 (6.5) | 100 (7.5) |
| 4 | H | Phenyl | 100 (7.5) | 200 (6.5) | 200 (5.5) | 200 (6.5) | 200 (7.0) | 200 (5.5) |
| 5 | CH3 | 4-Pyridinyl | 25 (6.5) | 50 (8.0) | 50 (5.0) | 200 (7.0) | 100 (7.0) | 100 (6.5) |
| 6 | CH3 | Phenyl | 100 (6.5) | 200 (7.5) | 200 (6.5) | 200 (7.5) | 200 (7.0) | 200 (8.5) |
| 7 | H | 4-Pyridinyl | 12.5 (6.0) | 25 (7.5) | 50 (6.0) | 100 (6.0) | 100 (6.5) | 50 (5.0) |
| 8 | CH3 | 4-Pyridinyl | 12.5 (6.5) | 25 (8.0) | 50 (7.0) | 100 (6.5) | 100 (7.0) | 100 (7.5) |
| 9 | H | Phenyl | 100 (7.5) | 200 (7.5) | 200 (8.0) | 100 (7.0) | 100 (7.0) | 100 (6.5) |
| 10 | CH3 | Phenyl | 50 (5.5) | 200 (8.5) | 200 (7.0) | 100 (7.5) | 100 (7.5) | 100 (7.5) |
| 11 | H | - | 50 (6.5) | 100 (5.5) | 100 (5.5) | 200 (6.0) | 200 (6.5) | 200 (5.8) |
| 12 | CH3 | - | 50 (5.8) | 100 (5.5) | 100 (5.5) | 200 (6.5) | 100 (7.0) | 100 (5.5) |
| 13 | H | H | 100 (6.5) | 200 (7.5) | 200 (7.0) | 50 (6.5) | 50 (6.5) | 100 (7.5) |
| 14 | H | CH3 | 100 (8.0) | 200 (5.5) | 200 (6.0) | 100 (8.5) | 50 (8.0) | 100 (7.5) |
| 15 | CH3 | H | 100 (6.0) | 200 (5.0) | 200 (5.5) | 50 (6.0) | 50 (7.0) | 100 (7.0) |
| 16 | CH3 | CH3 | 100 (6.0) | 200 (6.5) | 200 (5.5) | 100 (8.5) | 100 (8.0) | 100 (7.5) |
| 17 | H | - | 100 (6.5) | 200 (6.5) | 200 (7.5) | 100 (6.0) | 50 (6.5) | 50 (5.8) |
| 18 | CH3 | -- | 200 (6.6) | 200 (7.0) | 200 (7.5) | 100 (6.5) | 100 (7.0) | 50 (5.5) |
| 19 | H | SO2NH2 | 25 (7.5) | 25 (5.0) | 50 (5.5) | 100 (7.5) | 50 (8.0) | 25 (6.5) |
| 20 | CH3 | SO2NH2 | 25 (6.5) | 25 (6.5) | 25 (7.0) | 100 (6.0) | 25 (6.5) | 50 (8.0) |
| 21 | H | OH | 12.5 (7.5) | 12.5 (6.5) | 25 (6.5) | 50 (7.5) | 50 (6.0) | 25 (6.5) |
| 22 | CH3 | OH | 25 (8.5) | 25 (8.5) | 25 (7.5) | 50 (6.0) | 25 (6.5) | 25 (6.5) |
| 23 | H | H | 50 (7.5) | 50 (5.0) | 100 (5.5) | 25 (6.5) | 50 (8.0) | 25 (6.5) |
| 24 | H | NO2 | 25 (6.5) | 25 (6.5) | 50 (7.0) | 50 (8.0) | 50 (6.5) | 50 (8.0) |
| 25 | CH3 | H | 50 (8.5) | 50 (6.5) | 100 (6.5) | 25 (6.0) | 25 (6.0) | 50 (5.5) |
| 26 | CH3 | NO2 | 25 (6.0) | 25 (8.5) | 100 (7.5) | 50 (8.5) | 50 (6.5) | 50 (7.5) |
| Fluconazole | NT | NT | NT | 6.25 (9.5) | 6.25 (9.0) | 6.25 (8.5) | ||
| Ciprofloxacin | 6.25 (9.5) | 6.25 (9.0) | 6.25 (8.5) | NT | NT | NT | ||
| NT: denote Not tested, (-) absence of activity Table 1: Antimicrobial activity data of diversified 2-chloroquinoline derivatives (3-26) |
||||||||
| Compd. No. | Mol_Wt | WPSA | Volume | Donor HB | Accpt HB | QPlogP o/w | PSA | Rule of Five | Rule Of Three |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 310.742 | 64.226 | 962.235 | 1 | 5 | 3.074 | 73.511 | 0 | 0 |
| 4 | 309.754 | 64.117 | 976.411 | 1 | 3.5 | 4.094 | 60.754 | 0 | 0 |
| 5 | 324.769 | 64.169 | 1022.165 | 1 | 5 | 3.28 | 73.615 | 0 | 0 |
| 6 | 323.781 | 64.223 | 1037.317 | 1 | 3.5 | 4.409 | 60.804 | 0 | 1 |
| 7 | 308.726 | 57.971 | 928.031 | 0 | 5 | 2.927 | 61.591 | 0 | 0 |
| 8 | 322.753 | 57.971 | 989.286 | 0 | 5 | 3.261 | 61.591 | 0 | 0 |
| 9 | 307.738 | 57.533 | 942.958 | 0 | 3.5 | 3.985 | 48.683 | 0 | 0 |
| 10 | 321.765 | 57.533 | 1004.228 | 0 | 3.5 | 4.319 | 48.683 | 0 | 0 |
| 11 | 193.632 | 62.946 | 626.036 | 1 | 2.7 | 2.235 | 33.581 | 0 | 0 |
| 12 | 207.659 | 63.362 | 685.95 | 1 | 2.7 | 2.365 | 33.681 | 0 | 0 |
| 13 | 297.74 | 61.673 | 945.288 | 0 | 3 | 4.103 | 49.534 | 0 | 0 |
| 14 | 311.767 | 60.735 | 1004.841 | 0 | 3 | 4.413 | 49.494 | 0 | 0 |
| 15 | 311.767 | 57.832 | 1003.067 | 0 | 3 | 4.375 | 49.45 | 0 | 0 |
| 16 | 325.794 | 59.574 | 1064.683 | 0 | 3 | 4.727 | 49.527 | 0 | 1 |
| 17 | 212.078 | 131.419 | 646.807 | 0 | 1 | 3.53 | 11.915 | 0 | 0 |
| 18 | 226.105 | 131.228 | 706.306 | 0 | 1 | 3.982 | 11.907 | 0 | 0 |
| 19 | 347.818 | 64.174 | 1020.756 | 3 | 6.5 | 2.201 | 89.075 | 0 | 0 |
| 20 | 361.845 | 64.529 | 1080.092 | 3 | 6.5 | 2.482 | 89.077 | 0 | 0 |
| 21 | 284.744 | 62.903 | 907.097 | 2 | 2.75 | 3.7 | 46.299 | 0 | 0 |
| 22 | 298.771 | 62.742 | 967.929 | 2 | 2.75 | 4.009 | 46.303 | 0 | 0 |
| 23 | 325.815 | 98.236 | 986.45 | 1 | 2.5 | 4.878 | 37.828 | 0 | 1 |
| 24 | 370.812 | 98.186 | 1059.139 | 1 | 3.5 | 4.185 | 82.608 | 0 | 1 |
| 25 | 339.842 | 96.403 | 1046.061 | 1 | 2.5 | 5.169 | 38.381 | 1 | 1 |
| 26 | 384.839 | 97.713 | 1118.581 | 1 | 3.5 | 4.486 | 82.843 | 0 | 1 |
| Table 2: QikProp properties of all the compounds (3-26) calculated from QikProp tool of Schrodinger | |||||||||






































