Top Links
Journal of AIDS and HIV Infections
ISSN: 2454-499X
Role of Serology and Histopathology in Diagnostic of Human Cystic Echinococcosis
Copyright: © 2018 Albadawi AAM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Related article at Pubmed, Google Scholar
Hydatid disease is an important emerging neglected disease worldwide, with significant geographic variation in seroprevalance. The disease is commonly diagnosed on basis of clinical suspicion, imaging and serology. This study was conducted to evaluate our experience with serology in diagnosing hydatid disease in terms of identifying the numbers of patients testing positive for antibodies against Echinococcus, and to study their clinical profile. This study included all patients who tested for anti-Echinococcus IgG antibodies by ELISA in the years 2011-2013. The clinical characteristics of seropositive patients were contrasted with seronegative patients, and the test results were correlated with radiological findings and, where performed, with histopathological studies. Analysis was performed on SPSS. Out of 31 patients evaluated by serology for Echinococcosis during the study period, 7 (22.58%) were seropositive. Liver was the most frequent site involved (24 patients; 77.77%). Second organ the lung 5(16.13%) bone 1(3.23%) and the optic 1(3.22%). Of the 29 patients who also underwent biopsy, all the seven patients with positive biopsy were seropositive, suggesting 100% sensitivity. The specificity was 83.33%, but this might be a lower estimate due to potentially high rate of false-negative biopsies, as all the seropositive patients also had imaging features suggestive of disease. The positive and negative predictive values were 77.77% and 90.90%, respectively. ELISA for detection of antiEchinococcus antibodies is a simple serological test that helps in correlation with imaging finding in the diagnosis and subsequent management of hydatid disease.
Material and Methods: A cross sectional study was done on biological material (obtained from haptic and lung hydatid cysts). The viability criteria used were ovoid form invaginated scolices and intact calcareous corpuscles the presences of vibrating movements; and the absence of “vital” staining. The cysts were grouped according to diameter cysts from small cysts to very large size cysts. Descriptive statics for the collection of the prevalence of fertility; analytical statistics for comparison of groups, and multivariate analysis for examination of the association between cyst fertility and clinical variables. Is using to carried out by SPSS programme.
Results: Out of 31 patients evaluated by serology for Echinococcosis during the study period, 7 (22.58%) were seropositive. Liver was the most frequent site involved (24 patients; 77.77%). Second organ the lung 5(16.13%) bone 1(3.23%) and the optic 1(3.22%). Of the 29 patients who also underwent biopsy, all the seven patients with positive biopsy were seropositive, suggesting 100% sensitivity. The specificity was 83.33%, but this might be a lower estimate due to potentially high rate of false-negative biopsies, as all the seropositive patients also had imaging features suggestive of disease. The positive and negative predictive values were 77.77% and 90.90%, respectively.
Conclusion: Evaluation ELISA test in sensitivity and specificity, prevalence rates of the fertility of hydatid cysts and viability of protoscoleces and diversity sites location observed in human. And also the conformed diagnosis by histopathology.
Keywords: Human Cystic Echinococcosis; Microscopy; ELISA and histopathology; Sudan
Hydatidosis is a cycle-zoonotic parasitic infection caused by the Cestodes Echinococcus granulosus, (Lightowlers, et al, 2003) [1]. It is an important pathogenic, zoonotic and parasitic infection (acquired from animals) of humans, following ingestion of tapeworm eggs excreted in the faeces of infected dogs. And is a major endemic health problem in certain areas of the world (Moro P., Schantz PM. 2009 [2]; -Khanfar, N.; 2004[3]). The disease appears as multiple solid, tumor-like cysts sprouting on the interior and exterior of the organs. The cysts can range in size from 2 to 20 cm, are filled with fluid and can contain many smaller, daughter cysts (Romig; T. et al. 2011[4]). Cystic echinococcosis is globally distributed and found in every continent except Antarctica; (Echinococcosis, WHO, 2017) [5]; and also except Iceland and Greenland; The disease is characterized by an almost worldwide distribution causing significant morbidity and considerable socioeconomic impact in highly endemic regions; Significantly reducing meat and milk production as well as causing fertility loss in livestock. (Budke et al. 2013 [6], Budke CM, et al 2006 [7] ; Thoma, D. and Weible, A. K. 2006 [8], Jenkins, D. J., 2005 [9], Permin and Hanser, J. W., 2004 [10]). It is especially prevalent in parts of Eurasia, north and east Africa, Australia, and South America, Communities that practice sheep farming experience the highest risk to humans, (McManus DP,et al;2003) [11]. But wild animals can also serve as an avenue for transmission. For example, dingoes serve as a definitive host before larvae infect sheep in the mainland of Australia. (McManus DP,et al;2003) [ 11]. Sled dogs may expose moose or reindeer to E. granulosus in parts of North America and Eurasia. The incidence rate in hospitals is about 1000 times less than the prevalence as only a small proportion of patients who develop severe symptoms seeks medical care. (Grosso G, et al, 2015) [12]. As per the recommendation by the World Health Organization, hospital data can be used to measure the prevalence of CE in a population (Sadjjadi SM. 2006 [13]; Eckert, J. and Deplazes, P. 2004 [14]. Despommier, D,et al,2006 [15];Despommier, D,et al,2006 [15]). The presence of these isolated cases in man which is not really normal intermediate host for the hydatid cyst but still having our attention to the importance of hydatid cyst, which could be considered as that occurs in wild animals which are becoming relatively more important as that to human health (King and Hutchinson, 2007) [16].
The fertility of cysts is an important factor that can influence the transmission of E. granulosus. Depending on the nature of infected hosts and the sites of infection, cysts may have different fertility rates. These cysts commonly occur in the lungs and liver, but can be found in any other organ or tissue including bones, spleen, heart, eye, brain, and genitourinary tract. Anatomo clinical changes are peculiar to localization in the bone (Zlitini, M.et alk 2001) [17] From the anatomopathologic stand point, this localization marks the torpid, insidious progression of the parasite into the bone tissue, leading to a diffuse, extensive, invasive process; so from the clinical stand point, wherever it is localized, its complete surgical eradication is rarely possibly.(Zlitini, M.et al 2001) [17].
Hepatic cysts were more frequent than pulmonary cysts at ratio/or symptomatic cases (Lsrrieu and Finder, 2001; Djuricic; et al 2010 [18]), the factors that determine the final anatomic location of cysts are still unknown; however the liver is the organ most frequently infected because oncospheres penetrate the intestinal wall and tend to disseminate to liver via the portal vein. Variation in cyst location frequencies observed in diverse studies throughout the world could be due to infection with different species, (Pierangeli; et al; 2007) [19]. The germ membrane has less resistance to trauma, presents itself with a great number of nuclei and has a double role: helps the parasite reproduce and secretes the cyst’s liquid. This layer permits also the development of “daughter vesicles”. The hydatid sand found in the clear liquid has the immunological capacity of response, and both can generate the anaphylactic reaction of the host (Tenguria; RK, Naik; MI. 2014) [20].
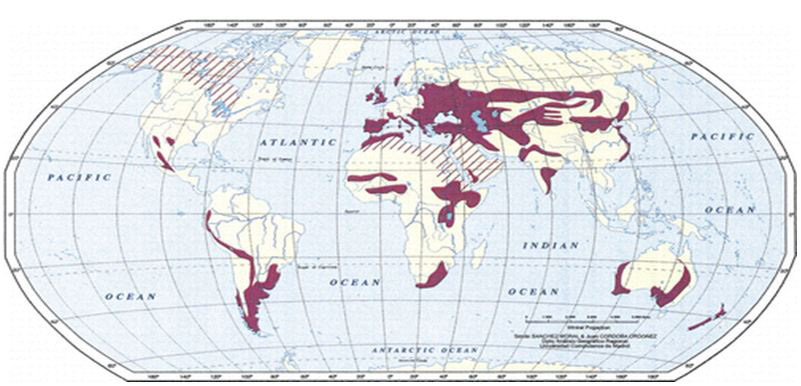
Figure 1. Geographic distribution of hydatid disease Map shows areas in which hydatid disease is endemic due to the transmission of E granulosus by means of the dog-sheep cycle (solid red areas). Red stripes indicate areas where transmission occurs by means of alternative life cycles in which carnivores such as wolves and foxes serve as definitive hosts and goats, camels, and horses serve as intermediate hosts. Transmission by means of alternative life cycles is common in North Africa, the Middle and Far East, the United States, Canada, and Iceland. E. granulosus is transmitted from the intermediate host (sheep) to the definitive host (dogs) by frequent feeding of offal, also referred to as “variety meat” or “organ meat”. Consuming offal containing E. granulosus can lead to infection; however, infection is dependent on many factors.
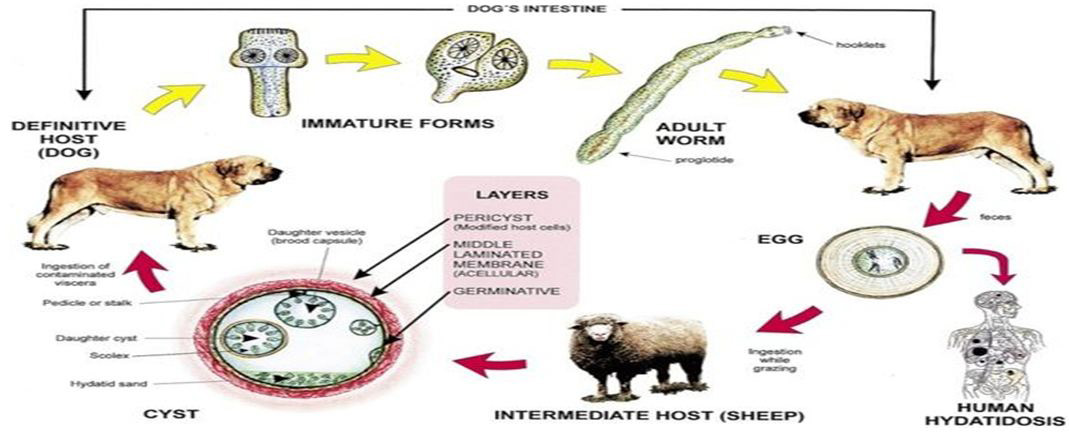
The lifecycle of E. granulosus involves dogs and wild carnivores as a definitive host for the adult tapeworm.(Moro P, Schantz PM (March 2009) [2]. Definitive hosts are where parasites reach maturity and reproduce. Wild or domesticated ungulates, such as sheep, serve as an intermediate host.( Moro P, Schantz PM ; March 2009 [2]; Torgerson PR, Heath DD 2003) [21]. Transitions between life stages occur in intermediate hosts. The larval stage results in the formation of echinococcal cysts in intermediate hosts. .( Moro P, Schantz PM (March 2009) [2]. Echinococcal cysts are slow growing, (Moro P, Schantz PM (March 2009) [2]. but can cause clinical symptoms in humans and be life-threatening. (McManus DP,et al; 2003 [11]) Cysts may not initially cause symptoms, in some cases for many years. (Moro P, Schantz PM (March 2009) [2]. Symptoms developed depend on location of the cyst, but most occur in the liver, lungs, or both. (McManus DP,et al; 2003) [11] When contaminated waste is excreted into the environment, intermediate host has the potential to contract the parasite by grazing in contaminated pasture, perpetuating the cycle. (Torgerson PR, Heath DD; 2003 [21]; Lahmar S,et al; 2004) [22]E. granulosus is transmitted from the intermediate host (sheep) to the definitive host (dogs) by frequent feeding of offal, also referred to as “variety meat” or “organ meat”. Consuming offal containing E. granulosus can lead to infection; however, infection is dependent on many factors. (McManus DP,et al; 2003) [11]
The life cycle of hydatid cyst; Image: CDC.Hydatidosis is known to occur in Sudan in man as well as animals but the extent of the disease in human population is not known. This is mainly due to the nonspecific clinical presentation and the nonexistence of sensitive and specific diagnostic methods, a part from confirmation at operation. Therefore this study was undertaken to study epidemiology of the disease among human population and to extract antigenic constituents from hydatid cysts in man, and attempt to analyses them and use them as targets for different serological tests for the laboratory diagnosis of hydatidosis in man.
1. To evaluate the sensitivity and specificity of ELISA test for detection of hydatid disease
2. To carry out the fertility of the cyst and viability of protoscoleces
1. To collect the cysts from internal organs (liver, lungs and other organs); and aspirate the fluid by centrifugation to separate the protoscoleces and large particles from the fluid for serology diagnosis.
2. Examination for macroscopic and microscopic examination for viable or non viable
Protoscoleces
3. To confirm histological examination of a suspected hydatid cyst with the serology and imaging techniques.
It is a cross sectional study, in biological material (the fluid from internal organ of the body of the human e.g. liver & lung).
The study area was conducted at the hospitals of Khartoum state from November 2011- July 2013.
Study period: This study was a analysis of suspected cases of hydatid disease who were tested for presence of IgG antibodies for Echinococcus granulosus by ELISA in years November 2011 - July 2013. Data was collected by attendant patients or suspected patients questionnaire information included (serial numbers; age; marital status; education and level of economic.
The assay used on all samples was an indirect Enzyme Linked Immunosorbent Assay (ELISA). Sensitivity and specificity of ELISA was calculated using histopathology as standard for those cases where histopathology results were available.
Plain radiography, ultrasound, CT, and magnetic resonance imaging (MRI) are the most useful diagnostic tools for detection of hydatid cystic lesions (Ataoglu H , et al 2002) [23]. CT and MRI in particular are highly sensitive for the diagnosis of hydatidosis and provide a complete lesion work-up ( Ataoglu H , et al 2002 [23], Hafsa C, et al, 2008 [24]). Magnetic resonance Imaging (MRI scan) - MRI delineates the cyst capsule better than CT scan, as a low intensity on both T1 and T2 weighted images. However, CT scan is better in demonstrating mural calcifications, Laboratory and imaging data are usually sufficient to establish a reliable diagnosis but sometimes they are inconclusive (Brunetti E. et al, 2010) [25].
CE diagnosis and monitoring firstly rely on imaging techniques. Ultrasonography (US) standardized classification of stage-specific cystic images has been issued by the WHO Informal Working Group on Echinococcosis (WHO-IWGE) for the diagnosis and the clinical management of CE (Brunetti,E; et al;2010) [25]. Serologic tests such as direct hemagglutination, latex agglutination, and immunoelectrophoresis are widely used to confirm the diagnosis; however, they have low sensitivity and specificity (Katilmiş H et al 2007 [26], Bouckaert MM, et al 2000 [27]), Raubenheimer EJ, Jacobs FJ.;(2000) [27]. Effective serological tests for CE diagnosis would be of great help to define and support cyst status and their evolution (active: CE1, CE2, and CE3b, transitional: CE3a, or inactive: CE4 and CE5) (Brunetti,E; et al;2010 [25], Hosch, W.et al; 2008) [28].
The technique was carried out on formalin fixed paraffin-embedded tissues (FFPT) from patients with histologically confirmed echinococcosis 2 sections (5 μm) were prepared from tissue blocks and excess paraffin was removed. Sections were placed in oven for dewaxen for 10 min at 37 °C. After dewaxen rehydration in 100%, 90%, 80% and 70% ethanol was followed by 70% ethanol for 3 min, then, water for 5 min. then stained in Mayer haematoxyline for 5 min. and bluing under tape water for 10 min and 3 minutes in eosin then rinsed 3 times in ascending alcohol and let to dry then mounted by DPX media.
Ethical approval for the study was obtained from the Ethical Committee of federal ministry of health, and permission was provided from all hospitals where investigation conducted in addition and informed consent was obtained from each participant prior to interview.
Data were analyzed by using the Statically Package for Social Science (SPSS)
Out of 31 patients evaluated by serology for Echinococcosis during the study period, 7 (22.58%) were seropositive. Liver was the most frequent site involved (24 patients; 77.77%). Second organ the lung 5 (16.13%) bone 1(3.23%) and the optic 1(3.22%).Of the 29 patients who also underwent biopsy, all the seven patients with positive biopsy were seropositive, suggesting 100% sensitivity. The specificity was 83.33%, but this might be a lower estimate due to potentially high rate of false-negative biopsies, as all the seropositive patients also had imaging features suggestive of disease. The positive and negative predictive values were 77.77% and 90.90%, respectively.
A total of 32 patients were suspected of clinically and/or radiologically suffering from hydatid disease during the study period, and tested for IgG antibodies against hydatid disease. The mean age of all the patients was 45 years (range: 14-80 years). Highest numbers of cases were seen among age group of 31-60 years. Males and females constituted a nearly identical number of patients with suspected hydatid disease (2 and 2, respectively). However, male patients were relatively younger compared to females, with a mean age of 38 and 45 years, respectively. Frequency distribution of age and gender is shown in Table 1).
Moreover, several studies have reported that the ultrasonography is more useful in diagnosing cystic Echinococcosis than CT and MRI (Wuestenberg et al., 2014 [29]; Stojkovic et al., 2012) [30]. When compared with CT scan, MRI has been shown to reproduce the findings of ultrasonography better (Stojkovic et al., 2012) [30].
Table 1 show hydatid cysts in different age group against gender
Table 2: show the hydatid cysts were diagnostic by radiography finding majority of the cases were detected by CT scan there were 26 cases and 5 cases by USG and 1 case by MRI. CT is indicated in cases in which US fails due to patient-related difficulties (eg, obesity, excessive intestinal gas, abdominal wall deformities, previous surgery) or disease complications. CT has a high sensitivity and specificity for hepatic hydatid disease. CT may display the same findings as US. Cyst fluid usually demonstrates water attenuation. Calcification of the cyst wall or internal septa is easily detected at CT. Ultrasonography is the screening method of choice. It is currently the primary diagnostic technique and has diagnostic accuracy of 90%.
Intravenous administration of contrast material is not necessary unless complications are suspected, especially infection and communication with the biliary tree (13).
During the course of each operation, the hydatid fluid of the lesion was removed through a trocar and stored separately in sterile receptacles at room temperature. All samples were processed with the same protocol within 2 hours after their extraction in the Parasitology Laboratory. The first step consisted of sedimentation of the hydatid fluid in order to separate the hydatid gravel. Subsequently samples were taken and subjected to wet mount observation under a light microscope at different powers to evaluate the general characteristic of the protoscoleces, Finally, ‘‘vital’’ staining was applied to the preparations by staining with an aqueous solution of 0.1% eosin from which proceeded a count of protoscoleces and the description of the morphological aspects.and some of the biopsy fixed in 10% formalin.
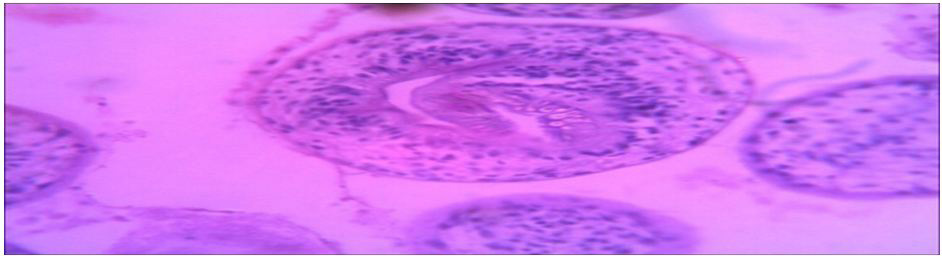
Histopathological characteristics of cystic echinococcosis lesions in humans. Cystic echinococcal lesion in the liver (HE stain×100); laminated layer; germinal layer; protoscolesis.
1. Specimen (1,4 and 5): Specimen (1) liver tissue, Specimens (4 and 5) lung tissue
Diagnosis: Hydatid cyst
Microscopy: section of specimens show cyst wall composed of fibrous laminated layer with inner germinal layer with broad capsule and scolices surrounded by fibrous capsule.
Diagnosis: Hydatid cyst
Microscopy: section of specimens show cyst wall composed of fibrous laminated layer with inner germinal layer with broad capsule and scolices surrounded by fibrous capsule.
Diagnosis: pyogenic cavity
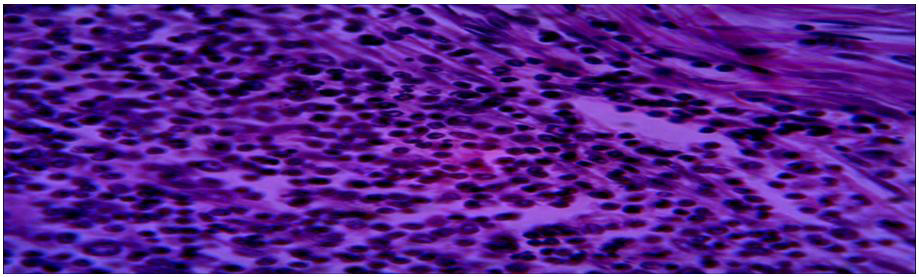
Microscopy: section show lung tissue with wall composed of inflammatory granulation tissue with suppuration. NO hydatid cyst.
Specimen (4): specimen (3) Lung tissue
Diagnosis: pyogenic cavity
Microscopy: section show lung tissue with wall composed of inflammatory granulation tissue with suppuration. No hydatid cyst.
Specimen (3) Lung tissue
Diagnosis: Hydatid cyst.
Microscopy: section show lung tissue fibrovascar tissue with inflammatory granulation tissue. No
Hydatid
This study was conducted on 31 patients of hydatid disease which included (35.48%) serodiagnosis using (purified antigen) of Echinococcus granulosus cyst fluid male patients most of them being farmers 15(48.38%) and female 16(51.61%) the maximum numbers of cases were housewives (Table 1). The liver was most common affected organ 24(77.42%), follow by lung 5(16.13%), bone 1(3.23%) and optic 1(3.22%) (Table 2). The higher rate of hepatic infection may be attributed to the fact that liver acts as primary filter in human body and lung is often thought to be the second filter.
Immunodiagnosis is an important tool for diagnosis of CE infection. Thus in addition to imaging techniques a reliable serodiagnosis improves prognosis for patients with cystic Echinococcus. The definitive diagnosis of cystic Echinococcusis by combination of CT scan or MRI with serological testing, the detection of circulating E. granulosus in sera is less sensitive than antibody detection which remains the method of choice. (Zhang, W. et al 2003) [31].insensitive and non-specific tests like Casoni intradermal test, complement fixation test, latex agglutination test and indirect haemagglutination test have been replaced by ELISA, indirect immunofluoresence antibody test, immunoelectrophoresis and immunoblotting (Lightowles MW, and Gottstein B. 1995) [32]. Among these ELISA for detection of IgG antibodies is most commonly used. It considered being highly sensitive and specific in detecting anti-Echinococcus antibodies irrespective of the site of cyst localization (Wattal C, et al, 1986) [33].
In similar study by Wattal et al, among purified antigens ELISA for IgG was the most sensitive (96.5%) in comparison to our study which showed almost sensitivity of 100% (Wattal C, et al, 1986) [33]. These results are similar to those obtained by (Kaddah et al 1992) [34]. The sensitivity and specificity of diagnosis of most of the tests varied considerably according to the nature, purity, and quality of antigen, according to the nature of the immunoglobulins (e.g. isotypes), and according to the sensitivity methodology chosen (Gottstein B. 1992) [35].the reported high sensitivity of tests using purified antigens was produced in this study in our laboratory. Moreover, several studies have reported that the ultrasonography is more useful in diagnosing cystic Echinococcosis than CT and MRI (Wuestenberg et al., 2014 [29]; Stojkovic et al., 2012) [30]. When compared with CT scan, MRI has been shown to reproduce the findings of ultrasonography better (Stojkovicet al., 2012) [30]. Immunological investigations are always considered complimentary to imaging in diagnosing Echinococcosis due to lack of sensitivity and specificity. Antibody response in case with Echinococcosis infection largely depends on various factors such as the stage of disease, site of involvement, etc. Therefore false negative results are seen in cases of small cysts, calcified cysts, extra hepatic cysts and cysts in privileged sites (Manzano-Román et al., 2015 [36]; Florea et al., 2011) [37]. Immunodiagnostic tests are also used for treatment monitoring in addition to primary diagnosis. IgG4 subset estimation is a good marker for treatment outcome; the levels decrease immediately after surgery and remain high in case of recurrent disease (Zhang et al., 2003) [31].Hydrated cyst fluid (HCF), extracts from protoscolises, larval forms and adult worms are potential antigenic sources for the serological diagnosis of hydatid disease. Antigen B and antigen 5 are the most common constituents of HCF which are used for development of immunological tests. Among these, antigen B is a better antigen as compared to antigen 5 with respect to cross reactivity (Sarkari et al., 2015) [37]. Detection of specific IgG subclasses have also shown to have diagnostic significance. Specific IgG4 isotype is shown to be associated with active disease and has been shown as a good prognostic marker in many studies (Sarkariet al., 2015 [37]; Manzano-Román et al., 2015 [36]).
Histopathological examination of a suspected hydatid cyst is confirmatory, but is not routinely performed, as imaging and serological tests are usually diagnostic, and it has been considered that biopsy harbors the risk of anaphylaxis, and dissemination of disease. However, it may be of benefit in confirming diagnosis when other tests are ambiguous, or in cases with presentation at unusual sites. More than half of the patients in our study underwent histopathological evaluation, of which only 7 were reported positive. In comparison with histopathology, serology had 100% sensitivity, but a lower measured specificity of 81%. However, it must be noted that all the 13 seropositive patients’ had evidence of hydatid cyst on imaging, suggesting a possibly increased false negative rate on histopatholgical evaluation due to unexplained reasons.
serology for detection of antibodies against hydatid disease is infrequently utilized. However, it is a simple test to confirm the diagnosis, and proved to be robust in our experience, with 100% sensitivity when compared with imaging and/or histopathology. The calculated specificity of 81.8% when compared to biopsy is likely an underestimation, as serology results correlated with imaging endings in all of the seropositive patients.
The authors declare that they have no competing interests.
AAMA was collected hydatid cysts, examined macroscopic and microscopic for the cysts, for the location, size, and is it fertile, also viability of protoscolsces and prepared the antigen. MHB was designed them and prepared the final manuscript, NTEO analyzed the final manuscript; MAM is director of the lab. Was helped with her experience, and AHG was competent and helped in the practical, HBO helped in the preparation of antigen.
Authors Would like to thanks the colleagues who helped me in performing this study particularly to their cooperation for collecting hydatid cysts samples from hospitals in Khartoum state. This study was made possible by invaluable assistance provided by Mr. Hatim Babeker Osman is director of Poilo Lab. At the authors are very grateful to Miss: Aida Hassan Gaber and Mawheb Abdelmoneim, Mycology Department, University of Khartoum.
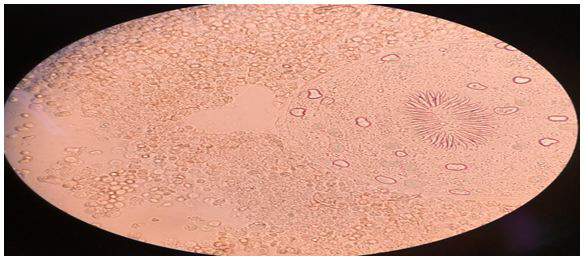
 |
 |
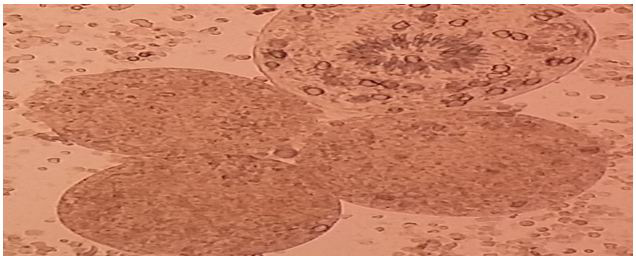
 |
 |
| Fig. 1: Wet a mount observation: Significant mass of ovoid protoscoleces can be seen, with their normal structures and calcareous corpuscles intact. |
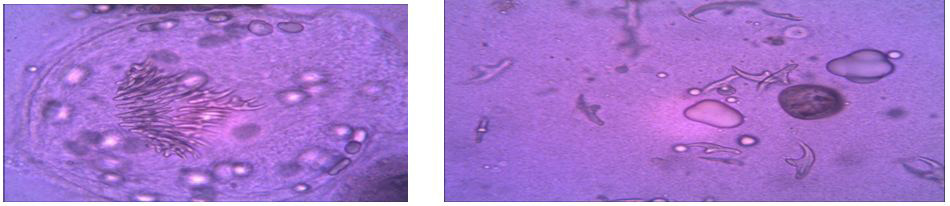 |
| Figure 2: shows the viable protoscoleces and hook let stain by staining with an aqueous solution of 0.1% eosin. |
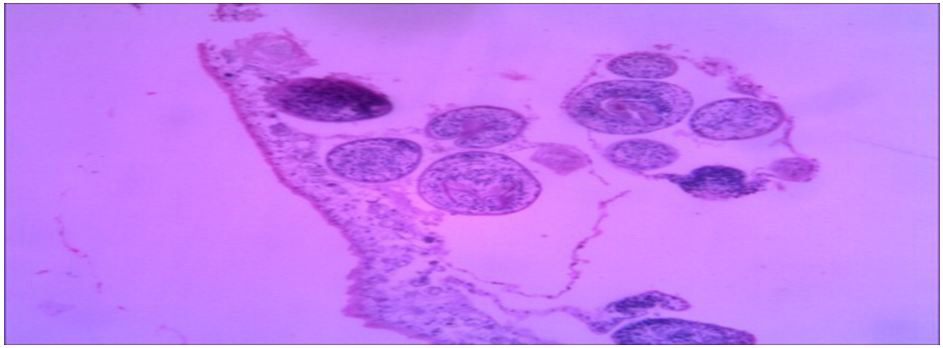 |
| Fig. (1) Section of specimen (1) lung tissue |
 |
| Fig. (2) Section of specimen (4&5) lung tissue |
 |
| Fig. (3) Section of specimen (2) lung tissue |
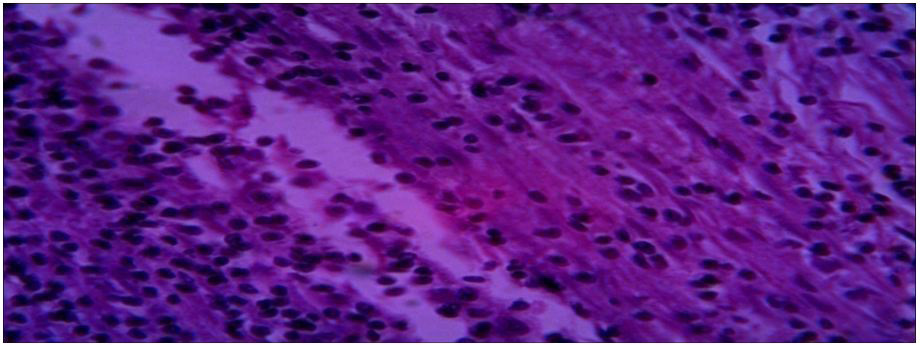 |
| Fig. (4) Section of specimen (3) lung tissue |
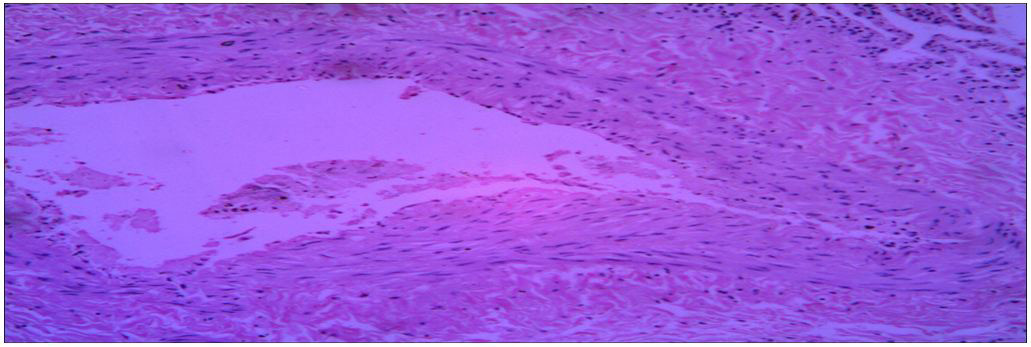 |
| Fig. (5) Section of specimen (6) lung tissue |
Organ |
Gender |
< 15 |
16- 30 |
31- 45 |
46 - 60 |
> 60 |
Total |
||||||
Liver |
Male |
|
1 |
2/1+ve |
4/1+ve |
4 |
11 |
||||||
female |
1+ve |
2/1+ve |
3 |
1 |
2 |
9 |
|||||||
Lung |
Male |
1+ve |
|
1 |
3 |
3 |
8 |
||||||
Female |
|
|
1+ve |
|
1 |
2 |
|||||||
Other origin |
Male |
|
|
|
|
1 |
1 |
||||||
female |
|
|
|
1+ve |
|
1 |
|||||||
Total |
|
2 |
3 |
7 |
9 |
11 |
32 |
||||||
Table 1:Comparison of laboratory parameters among patients with suspected Hydatid disease |
|||||||||||||
Origin |
Gender |
USG |
CT scan |
MRI |
Total |
||||||||
Liver |
Male |
3 |
8 |
|
11 |
||||||||
Female |
1 |
11 |
|
12 |
|||||||||
Lung |
Male |
1 |
2 |
|
3 |
||||||||
Female |
|
2 |
|
2 |
|||||||||
Other origins |
Male |
|
1 |
1 |
2 |
||||||||
Female |
|
2 |
|
2 |
|||||||||
Total |
|
5 |
26 |
1 |
32 |
||||||||
Table 2:Table shows hydatid cysts in different radiography finding. |
|||||||||||||






































